Take-home points
|
 |
Bios Dr. Park is a vitreoretinal surgery fellow at St. Michael's Hospital, University of Toronto. Dr. Chow is a vitreoretinal surgeon at Toronto Retina Institute and St. Michael's Hospital, and an assistant professor at University of Toronto. Disclosures: Dr. Park has no financial disclosures. Dr. Chow is a consultant for Alcon, Bayer, Roche, Aqueus, DORC and Aviceda. He conducts research for RegenXbio, Ophthea and Apellis. |
The discovery of anti-vascular endothelial growth factor treatments has been revolutionary in the world of ophthalmology and has paved the evolution of treatments for many retinal diseases. Aflibercept (Eylea, Regeneron), which was FDA-approved in 2011, is a VEGF decoy receptor and is effective in managing various retinal pathologies, including neovascular age-related macular degeneration, macular edema secondary to retinal vein occlusion and diabetic macular edema. Administered as an intravitreal injection of a 2-mg dose (0.05 mL of 40 mg/mL solution), the efficacy and safety profile of aflibercept has been demonstrated in multiple large-scale trials.
FDA approved in August 2023, Eylea HD is aflibercept administered intravitreally as an 8-mg dose (0.07 mL of 114.3 mg/mL solution), a four-times higher molar dose compared to its regular counterpart (2 mg). Recommended dosing frequency is every four weeks for the first three doses and every eight to 12 weeks (for DME) or eight to 16 weeks (for nAMD) thereafter. The approval was based on the two pivotal, multicenter, randomized, double-masked trials: PHOTON and PULSAR,1,2 the data of which were presented in 2023 at ARVO, ASRS and EURETINA meetings. Here, we’ll review the key findings of these studies.
PHOTON
The PHOTON trial compared 8-mg and 2-mg aflibercept in treatment of DME over a two-year period (96 weeks, n=658). Treatment arms consisted of:
• 2 mg every eight weeks after five initial monthly injections (2q8)
• 8 mg every 12 weeks after three initial monthly injections (8q12)
• 8 mg every 16 weeks after three initial monthly injections (8q16)
 |
|
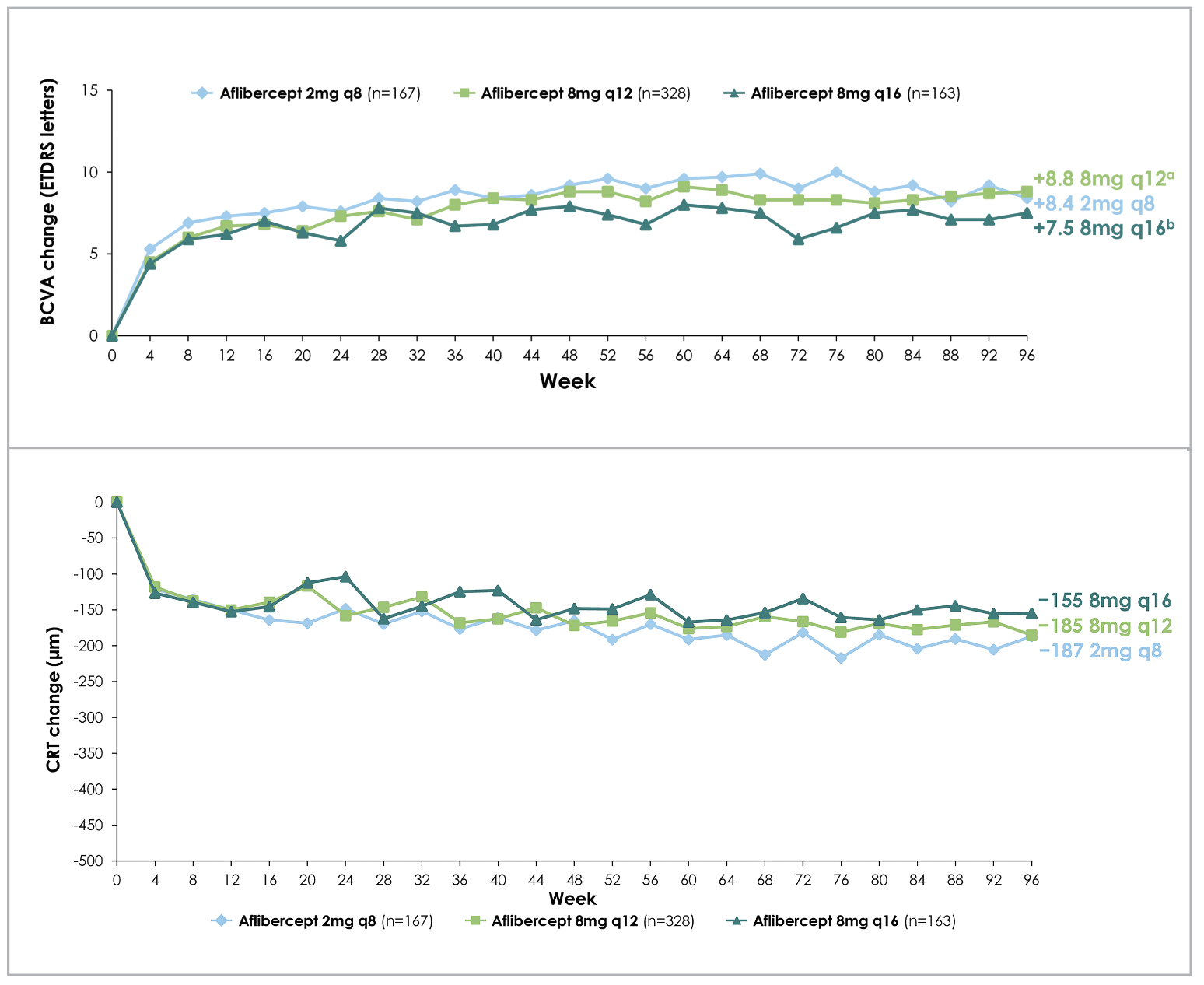
Figure 1. Mean change in best-corrected visual acuity from baseline (top), and central retinal thickness (bottom), in patients with diabetic macular edema with different aflibercept doses and intervals across two years (96 weeks), as per the PHOTON trial. |
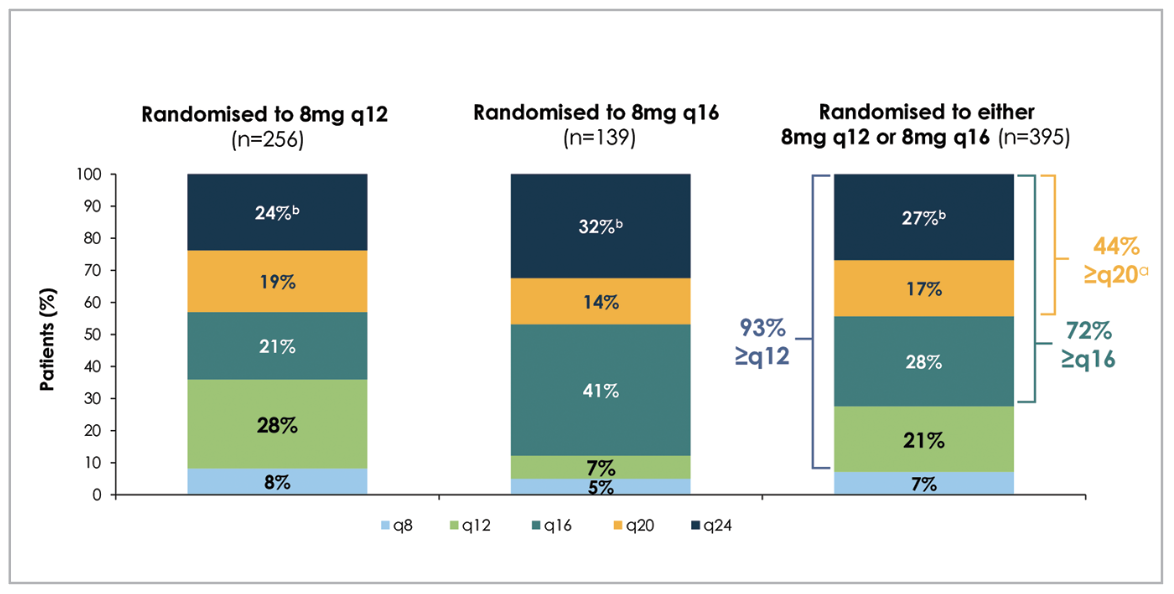
Despite fewer injections than the 2-mg arm (fewer initial monthly loading injections, and fewer total number of injections), the 8-mg groups maintained comparable improvements in vision and central retinal thickness (CRT) to the 2-mg group (Figure 1).3 Eighty-nine and 84 percent of patients were maintained on ≥12- and ≥16-week dosing intervals, respectively, throughout the two-year period, while sustaining their anatomic and vision improvements (Figure 2).3 Furthermore, as patients receiving 8-mg aflibercept were able to extend their dosing intervals in the second year (up to 24 weeks) by meeting pre-specified criteria, 44 percent met the requirements for ≥20-week intervals, including 27 percent who were eligible for 24-week dosing intervals.3 At 96 weeks, the mean number of injections were 13.8 for the 2q8 group, 9.5 for the 8q12 group and 7.8 for the 8q16 group.3
Subsequent subgroup analysis revealed similar BCVA gains from baseline between 8-mg and 2-mg groups regardless of age, sex, race and ethnicity.4 An interesting finding from ensuing univariable and multivariable analyses was that patients with more severe disease at baseline, specifically lower BCVA (BCVA ≤58 ETDRS letters) or higher central retinal thickness (CRT ≥474 µm), were more likely to need shortening of their intervals (down from 12- or 16-week) due to disease progression.5 However, despite the interval shortening, these patients were still able to achieve comparable BCVA and CRT improvements.
 |
|
Figure 2. Proportion of patients with diabetic macular edema with their last assigned aflibercept dosing and treatment intervals (in number of weeks) at the end of two years (96 weeks), as per the PHOTON trial. |
PULSAR
The PULSAR trial compared 8 mg and 2 mg aflibercept in treatment of nAMD over a two-year period (96 weeks, n=1,009). Treatment arms consisted of:
• 2 mg every eight weeks after three initial monthly injections (2q8)
• 8 mg every 12 weeks after three initial monthly injections (8q12)
• 8 mg every 16 weeks after three initial monthly injections (8q16)
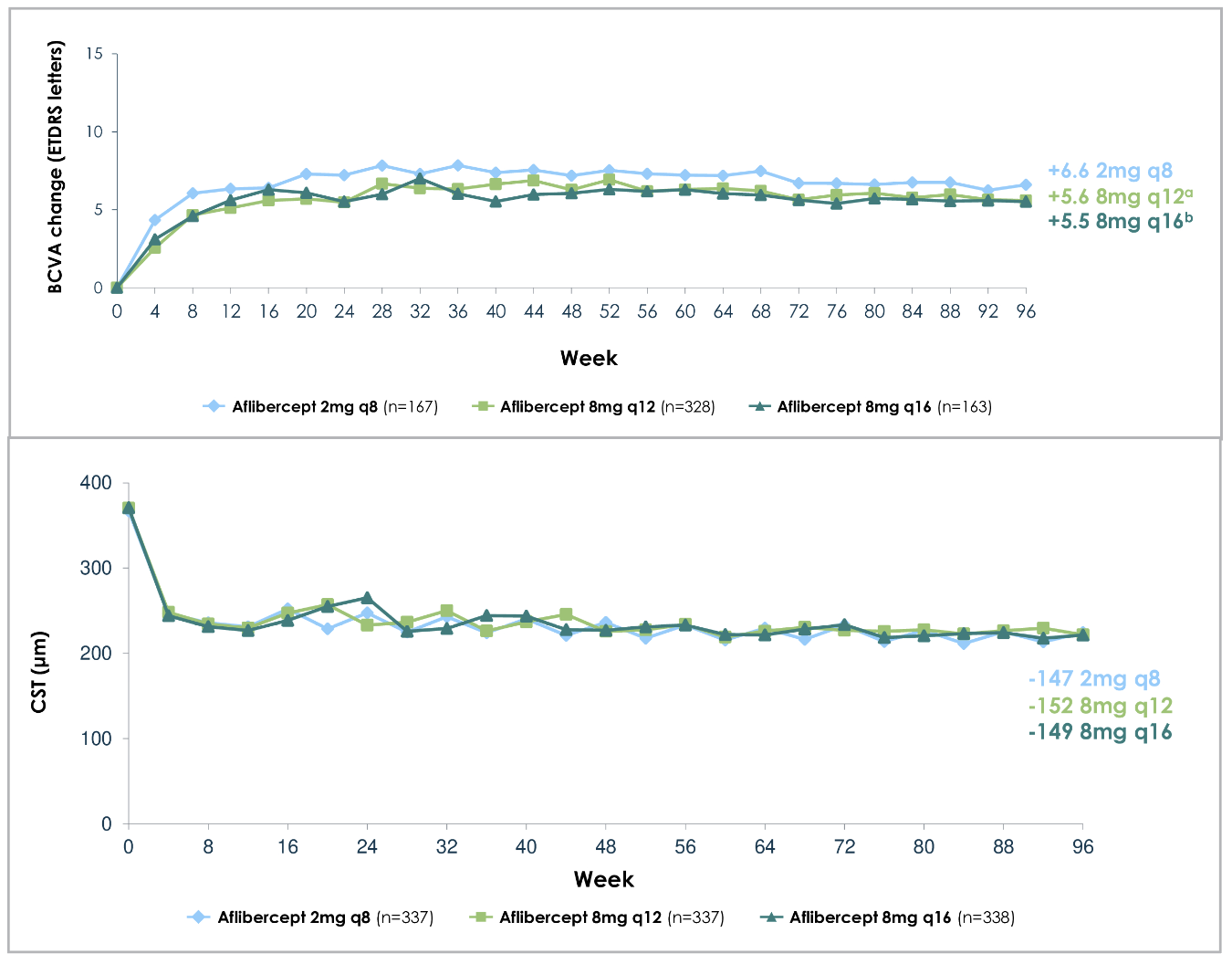
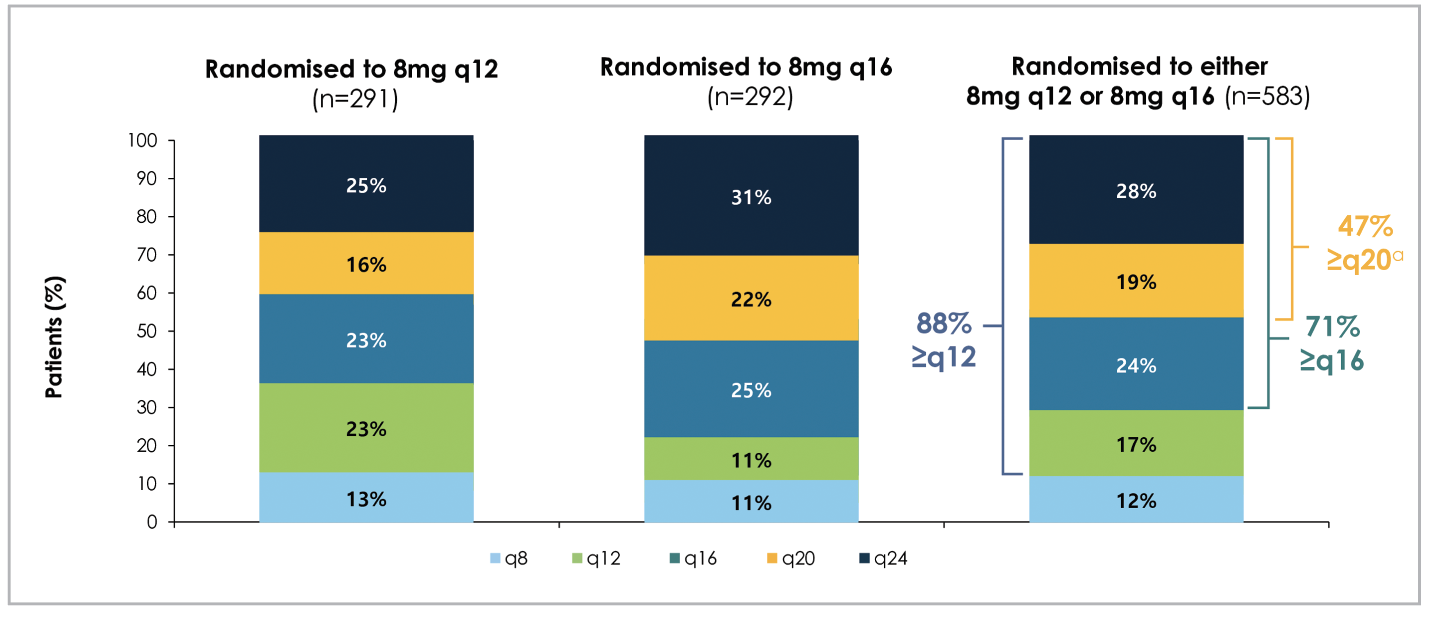
Similar to PHOTON’s results, PULSAR demonstrated durable vision gains and reduction in central subfield thickness (CST) with 8-mg aflibercept at extended dosing intervals that are comparable to the 2-mg group (Figure 3), with 88 percent treated on ≥12-week dosing interval at the end of two years (Figure 4).6 As with PHOTON, patients receiving 8-mg aflibercept in the PULSAR trial were able to extend their dosing intervals in the second year by meeting pre-specified criteria, and 71 percent met the extension criteria for ≥16-week dosing intervals, including 47 percent for ≥20-week intervals and 28 percent for 24-week intervals.6 At 96 weeks, the mean number of injections was 12.8 for the 2q8 group, 9.7 for the 8q12 group, and 8.2 for the 8q16 group.6
 |
|
Figure 3. Mean change in best-corrected visual acuity (BCVA) from baseline (top), and central subfield thickness (bottom), in patients with neovascular age-related macular degeneration (nAMD) with different aflibercept doses and intervals across two years (96 weeks), as per the PULSAR trial. ap=0.000 (nominal)(one-sided test for non-inferiority at four-letter margin vs. 2mg q8 group). bp=0.0007 (nominal)(one-sided test for non-inferiority at four-letter margin vs. 2mg q8 group). (Adapted from Lanzetta P, et al. Intravitreal aflibercept 8 mg injection in patients with neovascular age-related macular degeneration: 60-Week and 96-Week Results from the Phase III PULSAR trial. Presented at the European Society of Retina Specialists 2023 annual meeting, October 5-8, 2023.) |
Subgroup analysis showed consistent BCVA gains from baseline between 8 mg and 2 mg groups regardless of baseline BCVA, CST, choroidal neovascular membrane type (classic or occult) or race.7 Furthermore, post-hoc analysis revealed similar baseline BCVA, CST and CNVM size across all groups that maintained ≥12-week dosing intervals versus those that required interval shortening, which suggests the need for interval shortening may not be influenced by those baseline characteristics.8 A separate subgroup analysis comparing patients with polypoidal choroidal vasculopathy (PCV) to the overall population showed comparable improvements in BCVA and CST across all three treatment arms up to 48 weeks.9 Furthermore, no significant difference was found between PCV group and overall population with respect to being able to be maintained on a ≥12-week interval.9
 |
|
Figure 4. Proportion of patients with neovascular age-related macular degeneration with their last assigned aflibercept dosing and treatment intervals (in number of weeks) at the end of two years (96 weeks), as per the PULSAR trial. aDosing intervals were extended in Year 2 if participants met the following criteria: 1) less than 5-letter loss in BCVA from Week 12 AND 2) no fluid at the central subfield AND 3) no new foveal hemorrhage or neovascularization. (Adapted from Lanzetta P, et al. Intravitreal aflibercept 8 mg injection in patients with neovascular age-related macular degeneration: 60-week and 96-week results from the Phase III PULSAR Trial. Presented at the European Society of Retina Specialists 2023 annual meeting, October 5-8, 2023.) |
Safety
One of the proposed advantages of Eylea HD was that it provides a new treatment option that builds upon the established efficacy and safety profile of 2-mg aflibercept. Both PHOTON and PULSAR demonstrated a similar safety profile of the 8-mg aflibercept to the 2-mg aflibercept through the two years of the respective trials.3,6 Most common adverse reactions in the 8-mg aflibercept groups were cataract, conjunctival hemorrhage, increased intraocular pressure, ocular discomfort/pain/irritation, vision blurring, vitreous floaters, vitreous detachment, corneal epithelial defect and retinal hemorrhage.3,6
Pooled safety analysis performed with data across PHOTON, PULSAR and CANDELA, which was a Phase II, multicenter, randomized, single-masked study comparing 8-mg and 2-mg aflibercept doses for nAMD,10 also revealed comparable safety profiles between the 8 mg and 2 mg groups of aflibercept, with low incidence of intraocular inflammation.11 Serious adverse events were infrequent and consistent between the two doses: retinal detachment (0.4 percent and 0 percent); increased IOP (0.2 percent and 0 percent); and vitreous hemorrhage (0.2 percent and 0 percent) for aflibercept 8 mg and 2 mg, respectively.11 There were no cases of endophthalmitis or occlusive retinal vasculitis.11 There are limitations of the study in that it’s a pooled analysis and the data was limited to available data for the PHOTON, PULSAR and CANDELA trials, but the overall safety of 8-mg aflibercept appears to be consistent with the known safety profile of aflibercept 2 mg from previous clinical trials.
Bottom Line
The PHOTON and PULSAR trials demonstrated comparable visual and anatomical improvements between the 8-mg and 2-mg aflibercept groups in treating DME and nAMD, respectively.3,6 These benefits were sustained in the 8-mg group throughout the two years of trial duration despite the longer treatment intervals of every 12 or 16 weeks, which allowed for fewer total injections. Just as important, in both PHOTON and PULSAR, as well as the pooled safety analysis of PHOTON, PULSAR and CANDELA, the rate of adverse reactions in the 8-mg aflibercept group was infrequent and consistent with the known rate for the 2-mg aflibercept.3,6,10,11
Being able to unite improved clinical outcomes with reduction in treatment burden, all while not compromising safety, is an area of ongoing interest, and can make a significant impact in patient care. The 8-mg aflibercept clinical trials were the first to demonstrate that DME and nAMD patients can immediately be treated with every 12- or 16-week dosing intervals after their initial monthly doses and still experience clinically meaningful outcomes, while having a similar safety profile of the previously established profile of 2-mg aflibercept.
There are more questions that need to be addressed, such as the optimal dosing regimen, potential long-term implications of higher volume (0.07 mL) injections, as well as comparative data to other anti-VEGF agents, including recently approved faricimab (Vabysmo, Genentech). More research is warranted, and as the use of Eylea HD becomes more commonplace, more data is certainly expected, but the presented data thus far looks promising in improving patient care. RS
REFERENCES
1. ClinicalTrials.gov. Study of a high-dose aflibercept in participants with diabetic eye disease (PHOTON) (NCT04429503). Updated November 21, 2023. https://clinicaltrials.gov/study/NCT04429503. Accessed January 7, 2024.
2. ClinicalTrials.gov. Study of the effects of high dose aflibercept injected into the eye of patients with an age-related disorder that causes loss of vision due to growth of abnormal blood vessels at the back of the eye (PULSAR) (NCT04423718). Updated January 5, 2024. https://clinicaltrials.gov/study/NCT04423718. Accessed January 7, 2024.
3. Do DV. Aflibercept 8 mg for diabetic macular edema: 2-year results of the Phase 2/3 PHOTON Trial. Presented at American Society of Retina Specialists 2023 annual meeting, July 28-August 1, 2023. (Full presentation available on Regeneron’s website). https://investor.regeneron.com/static-files/c5aea914-bada-4d6f-8742-817e319b972f. Accessed January 7, 2024.
4. Ghorayeb G. Intravitreal aflibercept 8 mg for diabetic macular edema: Week 48 efficacy outcomes by baseline demographics in the Phase 2/3 PHOTON Trial [abstract]. Investigative Ophthalmology & Visual Science 2023;64:8:2707.
5. Brown DM. Baseline characteristics of patients who did or did not maintain 12-week and 16-week aflibercept 8 mg dosing intervals in the Phase 2/3 PHOTON Trial [abstract]. Investigative Ophthalmology & Visual Science 2023;64:8:2813.
6. Lanzetta P, Schulze A, Schmidt-Ott U, et al. Intravitreal aflibercept 8 mg injection in patients with neovascular age-related macular degeneration: 60-week and 96-week results from the Phase 3 PULSAR Trial. Presented at European Society of Retina Specialists 2023 annual meeting, October 5-8, 2023. Accessed January 7, 2024 (Full presentation available on Regeneron’s website). https://investor.regeneron.com/static-files/06016223-ff10-4fff-b9bb-3162f0ef27d9.
7. Sivaprasad S, Leal S, Machewitz T, et al. Subgroup analyses from the Phase 3 PULSAR trial of aflibercept 8 mg in patients with treatment-naive neovascular age-related macular degeneration [abstract]. Investigative Ophthalmology & Visual Science 2023;64:8:2238.
8. Lanzetta P, Leal S, Machewitz T, et al. Baseline characteristics of patients maintaining q12 and q16 dosing with aflibercept 8 mg vs patients with shortened treatment intervals: Phase 3 PULSAR post hoc analysis [abstract]. Investigative Ophthalmology & Visual Science 2023;64:8:2239.
9. Wong TY, Heier JS, Zhang X, et al. Intravitreal aflibercept 8 mg in patients with polypoidal choroidal vasculopathy: Phase 3 PULSAR Trial subgroup analysis [abstract]. Investigative Ophthalmology & Visual Science 2023;64:8:2240.
10. Wykoff CC, Brown DM, Reed K, et al. Effect of high-dose intravitreal aflibercept, 8 mg, in patients with neovascular age-related macular degeneration: The Phase 2 CANDELA randomized clinical trial. JAMA Ophthalmol 2023;141:9:834-842.
11. Schneider E. Pooled safety analysis of aflibercept 8 mg in the CANDELA, PHOTON, and PULSAR Trials [abstract]. Investigative Ophthalmology & Visual Science 2023;64:8:3724.



