| Seventh Annual Pipeline Report |
Take-home points
|
 |
How we compiled this listing This listing was compiled from company press releases and regulatory filings, published reports in the literature, searches on ClinicalTrials.gov and the European Union Clinical Trials Register (EudraCT), presentations at the American Academy of Ophthalmology Retina Subspecialty Day, American Society of Retina Specialists, Retina Society, Association for Research in Vision and Ophthalmology, EURETINA 2023, Eyecelerator and OIS Retina, supplemented with conversations with a multitude of clinical investigators and representatives of trial sponsors. |
| List of tables Investigative agents for nAMD, DME, DR and RVO, page 20 Investigative programs in geographic atrophy and dry AMD, page 23 Gene therapy trials in AMD, DR and DME, page 25 Investigative therapies for inherited retinal disease, page 26 Anti-VEGF biosimilars in clinical trials, page 28 Investigative device-based platforms, page 31 |
The Year of Geographic Atrophy lived up to its billing. This year’s list of investigative treatments for retinal disease is notable for three high-profile exits, two of which are for GA. The U.S. Food and Drug Administration last year approved pegcetacoplan (Syfovre, Apellis Pharmaceuticals) and avacincaptad pegol (Izervay, Iveric Bio/Astellas Pharma).
Another high-profile FDA approval was aflibercept 8 mg (Eylea HD, Regeneron Pharmaceuticals) for neovascular age-related macular degeneration, diabetic retinopathy and diabetic macular edema.
GA continues to be a robust area for innovation. This year’s listing of GA candidates has grown to 16 from 12 last year, with seven new programs joining the list.
While any number of programs drop from one year to the next, new entries inevitably more than make up for those exits. This year’s list includes 100 entries in total, 29 of which are new. Last year’s list consisted of 87 entries, 21 of which hadn’t appeared previously.
A number of candidates carried over from last year’s list appear under new names this year. For example, Xipere (Clearside Biomedical) is now listed as Arcatus/ARVN001 (Arctic Vision). Xiflam (InflammX) last year is Tonabersat this year. On the primary list, four preexisting listings have added new names.
A number of listings from last year didn’t make this year’s list. One is Conbercept (Chengdu Kanghong Biotechnology), an anti-VEGF agent long approved in China. All of its trials on ClinicalTrial.gov are either listed as “Unknown Status” or haven’t posted updates for more than a year.
Programs that have either been terminated or haven’t posted updates for more than a year, and their sponsors didn’t reply to queries by press time, include:
- GB-102 (Graybug Vision).
- ISTH0036 (Isarna Therapeutics).
- PAN-90806 (PanOptica).
- THR-149 and THR-687
(Oxurion). - GT005 (Gyroscope Therapeutics).
- AGTC-402 and rAAV2tYF-PR.7-hCNGB3 (Applied Genetics Technologies Corp.).
- Visomitin/SkQ1 (Mitotech).
- Retilux (PhotoOpTx).
This list consists of programs in human clinical trials.
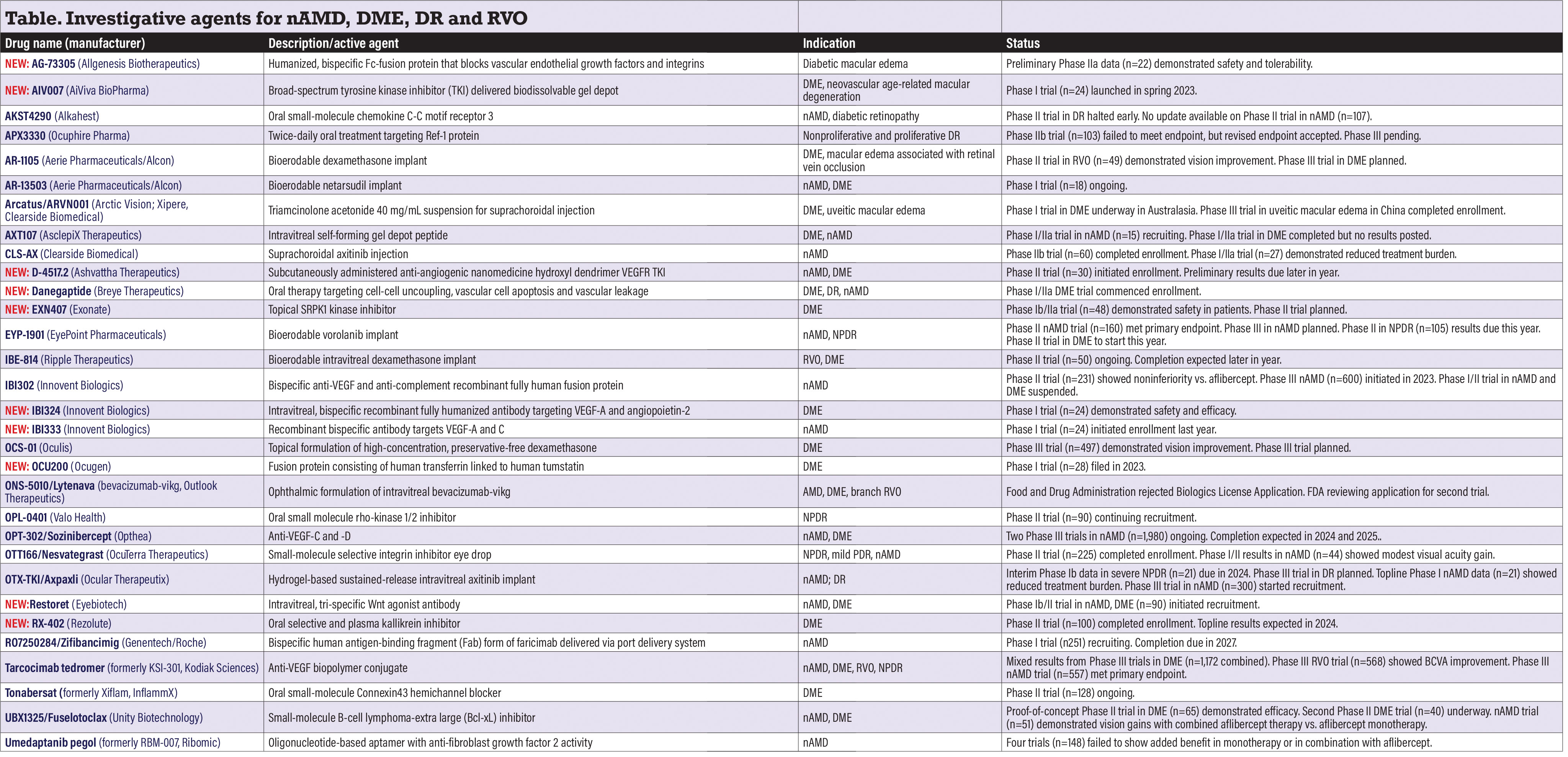
Investigative agents for nAMD, DME, DR and RVO
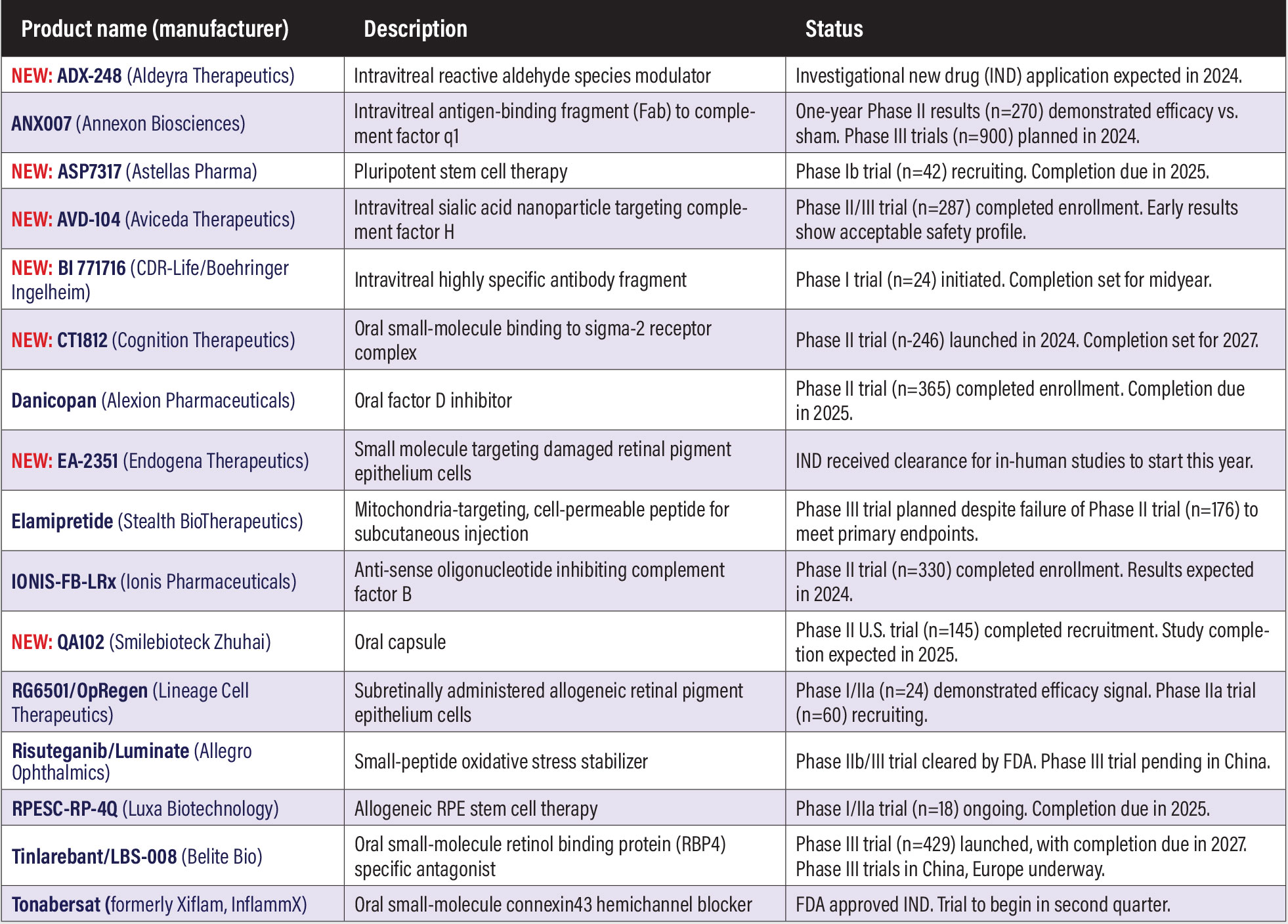
NEW: AG-73305 (Allgenesis Biotherapeutics)
This intravitreal humanized, bispecific Fc-fusion protein is designed to simultaneously block vascular endothelial growth factors and integrins. Preliminary Phase IIa data (n=22, NCT05301751) in DME reported no dose-limiting toxicities and no serious adverse events. Study completion is set for this year.
NEW: AIV007 (AiViva BioPharma)
Described as an injectable, broad-spectrum tyrosine kinase inhibitor (TKI), AIV007 is the focus of a Phase I trial initiated last year (n=24, NCT05698329). Subjects with DME and nAMD receive one periocular injection with monthly evaluation for up to six months. AIV007 uses a proprietary technology that transitions to a biodissolvable gel depot at body temperature for prolonged drug release. Study completion is set for 2025.
AKST4290 (Alkahest)
AKST4290 is an oral inhibitor of the chemokine C-C motif receptor 3 (CCR3) that blocks eotaxin, an immunomodulatory protein that increases as humans age and contributes to age-related diseases. The sponsor last year halted a Phase II trial in patients with diabetic retinopathy after enrolling just three patients (NCT04499235). A second Phase II trial in nAMD (n=107, NCT04331730), completed in 2021, evaluated oral AKST4290 daily in combination with aflibercept injections. Results were posted in 2022. Alkahest didn’t respond to a query for updated information.
APX3330 (Ocuphire Pharma)
This small-molecule, oral inhibitor blocks the downstream pathways regulated by the transcription factor regulator Ref-1 (reduction-oxidation effector factor-1), which involve VEGF and inflammation.
The Phase II ZETA-1 trial (n=103, NCT04692688) in moderately severe-to-severe nonproliferative DR or mild proliferative DR failed to meet its primary endpoint, but the U.S. Food and Drug Administration accepted a revised endpoint: the percentage of patients with greater than three-step worsening on a binocular Diabetic Retinopathy Severity Scale, which more placebo patients than APX3330-treated patients demonstrated. The FDA has green-lighted Phase III studies.
AR-1105, AR-13503 (Aerie Pharmaceuticals/Alcon)
AR-1105 is a dexamethasone implant platform for DME and retinal vein occlusion. Aerie reported last year that an open-label six-month study (n=49, NCT03739593) in patients with RVO-related macular edema demonstrated improvements in best-corrected visual acuity. Plans for Phase III trials in DME are underway.
AR-13503 is a rho-kinase inhibitor implant that’s an active metabolite of netarsudil that demonstrated efficacy as a monotherapy and anti-VEGF adjunct in preclinical studies. It’s the subject of a Phase I study in nAMD and DME (n=18, NCT03835884).
Arcatus/ARVN001 (Arctic Vision; Xipere, Clearside Biomedical)
This suprachoroidal triamcinolone acetonide suspension is already FDA approved for uveitic macular edema. Arctic Vision is pursuing the DME indication for its rebranded iteration in Australia, New Zealand and Asia. Arctic, meanwhile, has completed enrollment in a Phase III randomized, double-blind, placebo-controlled clinical trial in China in uveitic macular edema. No clinical trials are listed on ClinicalTrials.gov or the European Union Clinical Trials Register (EudraCT).
AXT107 (AsclepiX Therapeutics)
AXT107 is a microparticulate suspension for intraocular injection. It targets VEGF receptor 2 and activates the vessel-stabilizing receptor tyrosine kinase (Tie2). The sponsor is recruiting for a Phase I/IIa trial in nAMD (n=15, NCT05859776) that’s evaluating 40-week outcomes of three dose levels of a single injection. A Phase I/IIa trial in DME (n=6, NCT04697758) was completed in 2022, but no results have been posted.
NEW: AVD-104 (Aviceda Therapeutics)
Aviceda describes AVD-104 as an engineered glycan (sialic acid) nanoparticle that targets the self-pattern recognition receptors on overly activated retinal neutrophils, macrophages and microglia, and repolarizes them to their resolution state. Enrollment opened in the GLYCO Phase II U.S. trial (n=30, NCT06181227) in DME. Study completion is anticipated in the second quarter this year. Aviceda is also pursuing a Phase I trial in geographic atrophy.
CLS-AX (Clearside Biomedical)
CLS-AX is a suprachoroidal suspension of the TKI axitinib. Clearside completed enrollment in the Phase IIb ODYSSEY trial (n=60, NCT05891548), which randomized nAMD patients 2:1 to CLS-AX and aflibercept. Meanwhile, in the Phase I/IIa OASIS trial (n=27, NCT04626128) in nAMD, 83 percent of patients in the two highest dose cohorts (n=12) demonstrated reduction in treatment burden at four months. Completion of the ODYSSEY trial is due this year.1
NEW: D-4517.2 (Ashvattha Therapeutics)
D-4517.2 (hydroxyl dendrimer VEGF-R TKI) is a subcutaneously administered anti-angiogenic nanomedicine that selectively targets activated microglia, macrophages and hypertrophic retinal pigment epithelial cells. Enrollment started last year in a Phase II trial (n=30, NCT05387837) evaluating chronic dosing of D-4517.2 in nAMD and DME. The company expects to report preliminary results in the first half of the year.
NEW: Danegaptide (Breye Therapeutics)
This oral treatment for DR and DME targets cell-to-cell uncoupling, vascular cell apoptosis and vascular leakage at an early disease stage. Breye says it launched a Phase I/IIa trial in DME, but the only trials listed are in cardiovascular disease.
NEW: EXN407 (Exonate)
EXNP407 is a selective small-molecule drop designed to inhibit SRPK1 kinase to modulate VEGF splicing and rebalance VEGF isoforms to decrease angiogenic and pathogenic VEGF factors. A Phase Ib/IIa trial in mild NPDR (n=48, NCT0456756) demonstrated safety and a proclivity to reduce macular thickness and vascular leakage at 12 weeks compared with placebo, Exonate CEO Catherine Beech, MD, ChB, said at OIS Retina. The company plans to move forward to a Phase II trial.
EYP-1901 (EyePoint Pharmaceuticals)
This bioerodable sustained-release insert marries the Durasert platform with the TKI vorolanib. Topline data from the Phase II DAVIO 2 trial in nAMD (n=160, NCT05381948) showed noninferior BCVA improvement vs. aflibercept, along with no treatment-related serious adverse events. Patients in the 2- and 3-mg EYP-1901 dose groups demonstrated an 80-percent reduction in treatment burden.
A Phase III pivotal trial in nAMD should start enrollment in the second half of the year. Meanwhile, EyePoint says a topline data readout from the Phase II PAVIA trial in NPDR (n=105, NCT05381948) are expected in the second quarter, and the Phase II VERONA trial in DME should start recruitment in the first quarter this year.
IBE-814 (Ripple Therapeutics)
IBE-814 is a bioerodable, low-dose dexamethasone intravitreal implant that releases one-tenth the drug load of the corticosteroid dexamethasone. The Phase II trial in RVO and DME (n=60, NCT04576689) is continuing, with completion expected later in the year.
IBI302 (Innovent Biologics)
This intravitreal bispecific antibody targets both VEGF and C3b/C4b pathways. A Phase II comparator trial vs. aflibercept in nAMD (n=231, NCT04820452) reported comparable BCVA gains at 36 weeks.2 The Phase III STAR trial in nAMD (n=600, NCT05972473) is comparing IBI302 8 mg and aflibercept 2 mg. Enrollment opened last fall, with completion anticipated in 2027. A Phase I/II trial in both nAMD and DME (n=234) was suspended last year.
NEW: IBI 324 (Innovent Biologics)
This intravitreal, dual-target specific recombinant fully humanized antibody targets VEGF-A and angiopoietin-2 (Ang-2). Phase I results in DME (n=24, NCT05489718) demonstrated 4 mg was the maximum tolerated dose. No ocular serious adverse events, intraocular inflammation or dose-limiting toxicities were reported. Both single- and multiple-dose groups demonstrated BCVA improvement.3
NEW: IBI333 (Innovent Biologics)
This recombinant bispecific antibody targets VEGF-A and C. Enrollment opened last year in a Phase I nAMD trial (n=24, NCT05639530). IBI333 targets the VEGF-A-mediated signaling pathway to inhibit vascular endothelial cell proliferation and reduce VEGF-C-induced epithelial cell window formation to further reduce vascular permeability and inhibit the binding of compensatory up-regulated VEGF-C to endogenous VEGF receptors. Study completion is due this year.
OCS-01 (Oculis)
OCS-01 is a topical formulation of a 15-mg/ml-concentration of preservative-free dexamethasone. Results from Stage 1 of the Phase II/III DIAMOND trial (n=497, NCT05066997) in DME showed that patients on OCS-01 (n=100) had an average 7.6-letter improvement in BCVA at 12 weeks vs. 3.7 letters for those on vehicle (n=48).4 Patient enrollment has since started in the Phase III DIAMOND-1 trial, with completion expected in 2025.
NEW: OCU200 (Ocugen)
OCU200 is a novel fusion protein consisting of human transferrin linked to human tumstatin with antiproliferative, anti-inflammatory and antioxidative properties that selectively targets retinal and choroidal tissues. The formulation is designed to demonstrate better bioavailability and tissue penetration than tumstatin alone. Ocugen last year filed for a Phase I trial in DME (n=28, NCT05802329).
ONS-5010/Lytenava (bevacizumab-vikg, Outlook Therapeutics)
The FDA last year didn’t approve the biologics license application (BLA) for this ophthalmic formulation of Avastin. In December, Outlook submitted a special protocol assessment request with the FDA seeking confirmation that the NORSE EIGHT trial would fulfill the FDA’s requirement for a second clinical trial in nAMD. Outlook says it expects a response early this year. NORSE EIGHT would enroll about 400 patients and compare ONS-5010 1.25 mg with ranibizumab 0.5 mg. The previous NDA was based on Phase III NORSE TWO results (n=228, NCT03834753) in nAMD, which showed noninferiority vs. ranibizumab.
OPL-0401 (Valo Health)
OPL-0401 is an oral, small-molecule rho-kinase 1/2 inhibitor. The Phase II Spectra trial in mild, moderate and severe NPDR (n=90, NCT05393284) is continuing to recruit. Trial completion is set for this year.
OPT-302/Sozinibercept (Opthea)
OPT-302 targets VEGF-C and D. Opthea adopted sozinibercept as its nonproprietary drug name. Recruitment is continuing in two Phase III trials in nAMD: ShORe (n=990, NCT04757610) and COAST (n=990, NCT04757636). They’re evaluating intravitreal OPT-302 2 mg in combination with ranibizumab 0.5 mg or aflibercept 2 mg, respectively. The primary endpoint for both studies is superiority in visual acuity gains at 12 months for the combination therapy compared with monotherapy. Study completion is set for the end of this year in ShORe and next year for COAST. Phase IIb results demonstrated that combination OPT-302-ranibizumab for nAMD achieved a statistically superior gain in visual acuity at 24 weeks vs. ranibizumab alone.5
OTT166/Nesvategrast (OcuTerra Therapeutics)
Nesvategrast is the new name given this topical small-molecule, selective integrin inhibitor. OcuTerra completed patient enrollment in the Phase II DR:EAM trial (for Diabetic Retinopathy: Early Active Management) in adults with moderately severe-to-severe NPDR or mild PDR with minimal vision loss (n=225, NCT05409235). OcuTerra says it expects topline results in the first quarter this year. Results from the Phase I/II trial in nAMD (n=44, NCT02914639), posted last year, demonstrated good outcomes for safety and tolerability, but mixed outcomes for back-of-the-eye findings, along with modest improvement in BCVA improvement.
OTX-TKI/Axpaxli (Ocular Therapeutix)
This axitinib intravitreal implant also has a new name. Ocular reported completing enrollment in the Phase Ib trial in moderately severe-to-severe NPDR (n=21, NCT05695417). Interim six-month data are expected in the first quarter, when, Ocular says, it plans to also prepare a Phase III trial in DR. Twelve-month topline data from the Phase I nAMD trial (n=21, NCT04989699) demonstrated an overall 89-percent reduction in treatment burden in OTX-TKI patients.6 Ocular last year initiated the pivotal Phase III trial in nAMD (n=300, NCT06223958), for which the FDA this year granted a special protocol assessment agreement modification to include treatment-naive patients with VA of 20/80 or better.
NEW: Restoret (Eyebiotech)
This intravitreal, tri-specific agonist antibody targets the Wnt signaling pathway to halt vascular leakage and restore and maintain the blood-retinal barrier. The Phase Ib/II AMARONE trial (for Anti-permeability Mechanism and Age Related Ocular Neovascularization Evaluation) last year started enrolling patients with DME and nAMD. The trial isn’t yet listed.
NEW: RZ-402 (Rezolute)
RZ402 is an oral selective and plasma kallikrein inhibitor (PKI). Rezolute last year reported completing enrollment in the Phase II trial in DME (n=100, NCT05712720). The proof-of-concept study is evaluating RZ402 administered once daily as monotherapy over 12 weeks plus a four-week follow-up in treatment-naive patients or those who have had limited anti-VEGF injections. Topline results are expected in the second quarter this year.
RO7250284/zifibancimig (Genentech/Roche)
And yet another new name, this one for this bispecific human Fab form of faricimab delivered via the port-delivery implant used with Susvimo. The three-part Phase I BURGUNDY trial in nAMD (n=251, NCT04567303) is still recruiting patients. Study completion is set for 2027.
Tarcocimab (formerly KSI-301, Kodiak Sciences)
This anti-VEGF biopolymer conjugate has had quite the up-and-down year. Last summer, Kodiak said it would drop the program after the GLEAM (n=460, NCT04611152) and GLIMMER (n=459, NCT04603937) DME trials failed to show noninferiority vs. aflibercept. Kodiak pulled an about-face after first-time results from the GLOW Phase III trial (n=253, NCT05066230) showed 41 percent of treated patients had a greater-than-two-step improvement in DRSS score at 48 weeks vs. 1.4 percent of sham patients (p<0.0001).7 Tarcocimab patients received 3.8 injections a year on average.
Updated one-year results from the BEACON Phase III trial in RVO (n=568, NCT04592419) demonstrated tarcocimab met the primary endpoint of noninferiority vs. aflibercept for BCVA change at 24 weeks. Enrollment was also completed in the Phase III GLOW trial in NPDR (n=253, NCT05066230), but then the study was terminated because the primary endpoint wasn’t met.
The Phase III DAYLIGHT trial evaluating monthly tarcocimab in nAMD (n=557, NCT04964089) met its primary endpoint of noninferior VA outcomes vs. aflibercept.
Tonabersat (formerly Xiflam, InflammX)
This oral, small-molecule NLRP3 inflammasome inhibitor targets the Connexin43 protein and blocks the formation of hemichannels. A Phase II trial in DME (n=128, NCT05727891) is ongoing. The trial is also evaluating the therapeutic effect of Tonabersat on biomarkers of active kidney disease. Completion is scheduled for yearend.
UBX1325/Fuselotoclax (UNITY Biotechnology)
Here’s yet another new name. UBX1325 is a potent small-molecule inhibitor of B-cell lymphoma-extra-large (Bcl-xL) that aims to inhibit the function of proteins that senescent cells rely on for survival. The first patients were dosed in the Phase II ASPIRE study (n=40, NCT06011798) in DME. Study completion is anticipated at yearend. Forty-eight week results from the Phase II BEHOLD trial in DME (n=65, NCT04857996) demonstrated a 6.2-letter BCVA gain from baseline after one injection and led to about 50 percent of patients going without rescue injections.8
In nAMD, Part B of the Phase II ENVISION study (n=51, NCT05275205) enrolled patients who had failed on previous anti-VEGF therapy. Patients switched from aflibercept every eight weeks to a combination of aflibercept and UBX1325 at week 24 maintained vision gains achieved with aflibercept alone through week 48. In the UBX1325 monotherapy arm, patients maintained VA for the duration of the study. Forty percent of UBX1325 patients didn’t need anti-VEGF rescue through 48 weeks and 64 percent achieved an anti-VEGF treatment-free period of over 24 weeks.
Umedaptanib pegol (formerly RBM-007, Ribomic)
Umedaptanib pegol is an anti-fibroblast growth factor-2 aptamer that’s the focus of four clinical trials in nAMD: the Phase I SUSHI trial (n=9, NCT03633084);9 and three Phase II trials (TOFU, n=94, NCT04200248; RAMEN, n=40, NCT04640272; and TEMPURA, n=5, NCT04895293). TOFU evaluated intravitreal umedaptanib pegol monotherapy and combination therapy with aflibercept alongside aflibercept monotherapy.10
RAMEN is an extension study of TOFU subjects who received four monthly umedaptanib pegol injections. While the studies demonstrated safety and what Ribomic describes as “striking” improvements in BCVA and anatomy in some treatment-naive patients, umedaptanib pegol showed no added benefit either alone or in combination with aflibercept. The company says it needs to evaluate umedaptanib pegol in early nAMD and alongside anti-VEGF agents.
 |
| Click image to view table in the digital edition. |
REFERENCES
1. Brown DM. Safety and tolerability of CLS-AX via suprachoroidal injection in nAMD patients with persistent activity following anti-VEGF therapy. Paper presented at Retina Society. October 13, 2023. New York, NY.
2. Sun X. A Phase 2 study of IBI302, an anti-VEGF-anti-complement dual targeted drug, for the treatment of neovascular age-related macular degeneration (nAMD). Poster PO509 presented at the American Academy of Ophthalmology; November 3, 2023; San Francisco, CA.
3. Sun X. Phase 1 Study of IBI324 (anti-VEGF-A/Ang-2 bispecific antibody) for diabetic macular edema (DME). Poster PO524 presented at American Academy of Ophthalmology; November 3, 2023; San Francisco, CA.
4. Tadayoni R, Boyer DS, Markabi S, Dehmel B, Dugel P, Khanani AM, on behalf of the DIAMONG (DX-219) study group. A 12-week Phase 2/3 double-masked, randomized, multicenter study of OCS-01 OPTIREACH technology topical dexamethasone eye drops in subjects with diabetic macular edema (DME): Efficacy and safety findings. Paper presented at EURETINA; October 5, 2023; Amsterdam.
5. Jackson TL, Slakter J. Buyse M, et al, for the Opthea Study Group Investigators. A randomized controlled trial of OPT-302, a VEGF-C/D inhibitor for neovascular age-related macular degeneration. Ophthalmology. 2023;130:588-597.
6. Khanani AM. 12-Month update on randomized, controlled, trial of OTX-TKI (axitinib intravitreal implant) for the treatment of wet AMD. Paper presented at Clinical Trials at the Summit; June 10, 2023; Park City, UT.
7. Wykoff CC. Tarcocimab tedromer for diabetic retinopathy: Primary endpoint efficacy and safety outcomes of the GLOW Phase 3 Pivotal Study. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day. November 3, 2023; San Francisco, CA.
8. Sheth V. UBX1325 A novel senolytic candidate for patients with diabetic macular edema: 48-weeks results for BEHOLD Phase 2 study. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day. November 3, 2023; San Francisco, CA.
9. Pereira DS, Akita K, Bhisitkul RB, et al. Safety and tolerability of intravitreal umedaptanib pegol (anti-FGF2) for neovascular age-related macular degeneration (nAMD): A Phase 1, open label study. Eye. published online December 1, 2023. Available at: https://www.nature.com/articles/s41433-023-02849-6.pdf. Accessed January 23, 2024.
10. Pereira DS, Maturi RK, Akita K, et al. Clinical proof of concept for anti-FGF2 therapy in exudative age-related macular degeneration (nAMD): Phase 2 trials in treatment-naïve and anti-VEGF pretreated patients. Eye. Published online November 30, 2023. Available at: https://www.nature.com/articles/s41433-023-02848-7.pdf. Accessed January 23, 2024.
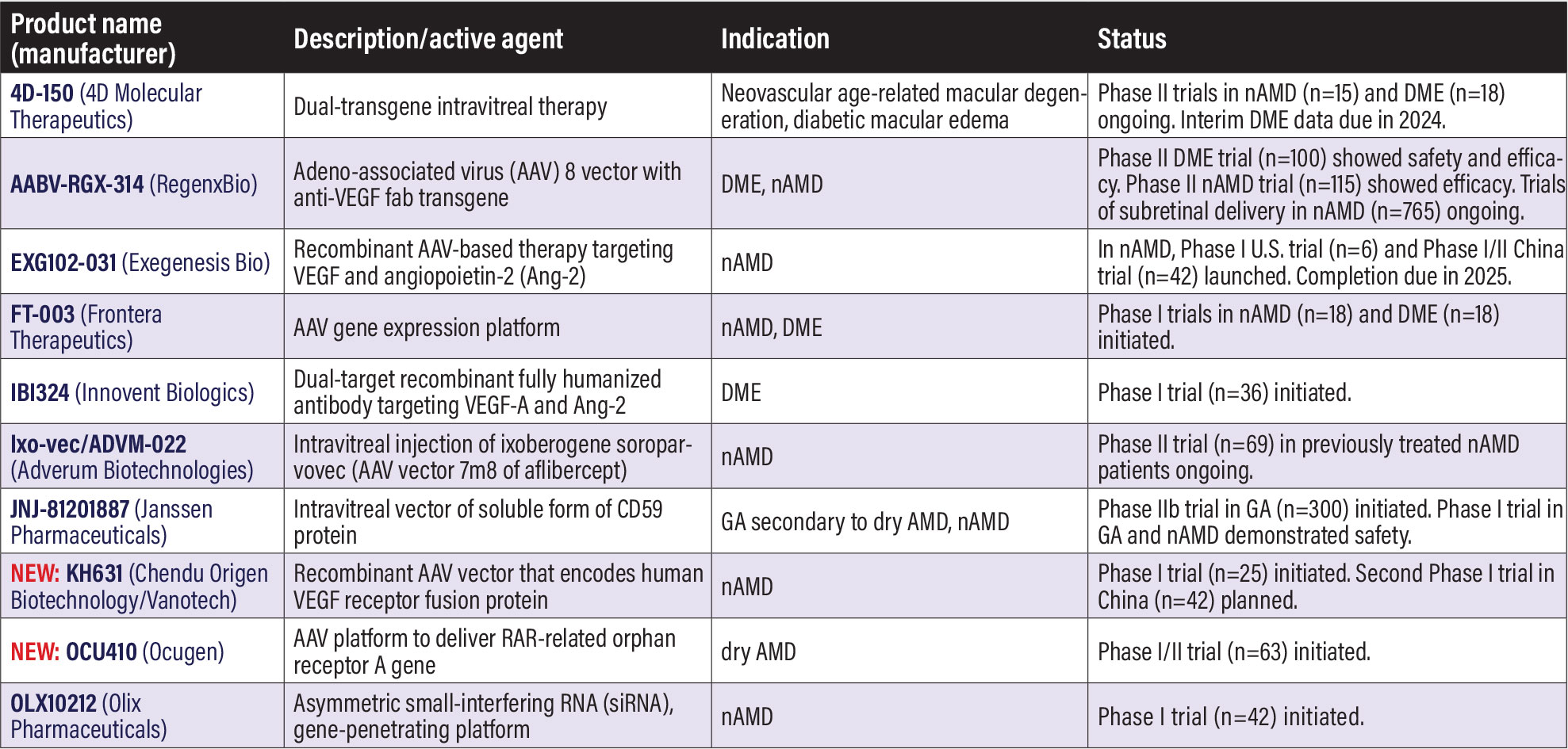
Gene therapy programs in AMD, DR, DME 4D-150 (4D Molecular Therapeutics). 4D-150 consists of the targeted and evolved intravitreal vector, R100, and a dual transgene payload that expresses aflibercept and a vascular endothelial growth factor-C RNA interference to inhibit VEGF A, B, C and placental growth factor (PLGF). It’s designed for low-dose intravitreal delivery. The first patients were enrolled last year in the dose-confirmation stage (n=18) of the Phase II SPECTRA trial (n=72, NCT05930561) in diabetic macular edema and in the population extension cohort of the Phase II PRISM trial (n=150, NCT05197270) in neovascular age-related macular degeneration. 4DMT says it expects to complete enrollment in SPECTRA and report interim 24-week data later in the year. 4D-150 also received regenerative medicine advanced therapy (RMAT) designation from the U.S. Food and Drug Administration for intravitreal treatment of nAMD. RMAT allows for accelerated review based on surrogate endpoints. Interim PRISM data demonstrated what 4DMT describes as “encouraging safety, tolerability and clinical activity” in nAMD. 4DMT this year expects to report interim PRISM data and update plans for the Phase III trial. AABV-RGX-314 (RegenxBio/AbbVie). This adeno-associated virus-8 (AAV8) vector contains an anti-VEGF transgene delivered suprachoroidally. One-year results from the Phase II ALTITUDE trial in DME (n=100, NCT04567550) showed acceptable tolerance in 50 patients, with 70.8 percent of treated patients achieving improvement in Diabetic Retinopathy Severity Scale scores vs. 25 percent of controls.1 In nAMD, interim six-month results of 65 patients in the Phase II AAVIATE trial (n=115, NCT04514653) demonstrated an 85-percent reduction in the annualized injection rate in the highest-dose cohort, with 67 percent remaining injection free.2 Both ALTITUDE and AAVIATE are due for completion this year. RegenxBio is also evaluating a subretinal delivery platform. The Phase II/III ATMOSPHERE (n=300, NCT04704921) and ASCENT (n=465, NCT05407636) trials in nAMD are expected to support a biologics license application (BLA) in 2025 or 2026. EXG102-031 (Exegenesis Bio). EXG102-031 is an injectable recombinant AAV-based gene therapy that aims to express a therapeutic fusion protein that binds or neutralizes all known subtypes of VEGF and angiopoietin-2 (ang-2). Two separate clinical trials in nAMD last year started recruiting patients: a Phase I U.S. trial (n=6) known as Everest; and a Phase I/II trial in China (n=42, NCT06183814). FT-003 (Frontera Therapeutics). FT-003 is an AAV gene platform. The first patients were dosed last year in two Phase I trials in China: in nAMD (n=18, NCT05611424); and in central-involvement DME (n=18, NCT05916391). Ixo-vec/ADVM-022 (Adverum Biotechnologies). The long name is isoberogene soroparvovec. Adverum completed enrollment last year in the Phase II Luna trial in nAMD (n=69, NCT05536973), randomizing patients to two different doses: a 2 x 1011 vector genes per eye (vg/eye) and a lower 6 x 1010 vg/eye dose. Patients are also randomized across four prophylactic regimens, with the goal to determine the utility of oral prophylaxis in future trials. JNJ-81201887 (Janssen Pharmaceuticals). This candidate has also been known as JNJ-1887, HMR59, developed by Hemera Biosciences, and AAVCAGsCD59. The intravitreal vector aims to boost expression of soluble CD59, a protective protein found in the cellular plasma membrane. The Phase IIb trial in GA, known as PARASOL, (n=300, NCT05811351) initiated recruitment last year. A pooled analysis of the open-label Phase I trial (n=17, NCT03144999) demonstrated a single injection was well-tolerated with an acceptable safety and a manageable inflammatory profile in patients with both GA and nAMD.3 NEW: KH631 (Chengdu Origen Biotechnology/Vanotech). This recombinant AAV vector encodes a human VEGF receptor fusion protein. The first patient was dosed in the VAN-2201 Phase I trial in nAMD (n=25, NCT05657301). A second Phase I trial is registered in China (n=42, NCT05672121). NEW: OCU410 (Ocugen). OCU410, also known as AAV-RORA, uses an AAV platform to deliver the RORA (RAR-related orphan receptor A) gene to the retina. The RORA protein is thought to reduce lipofuscin deposits and oxidative stress, and it has demonstrated anti-inflammatory properties in animal studies. Ocugen last year dosed the first patient in the Phase I/II ArMaDa trial in dry AMD (n=63, NCT06018558). OLX10212 (Olix Pharmaceuticals). This agent uses an asymmetric small-interfering RNA (siRNA), gene-penetrating technology to target genetic origins of inflammation. A Phase I nAMD trial (n=42, NCT05643118) launched last year. Early results demonstrate no serious safety signals. The study also identified dose levels suitable for future trials. In the study, OLX10212 was administered at dose levels between 100 µg/eye/50 µL and 950 µg/eye/50 µL via a single intravitreal injection. Trial completion is anticipated by yearend.
REFERENCES Barakat MR. Suprachoroidal delivery of investigational AABV-RGX-314 for diabetic retinopathy: The Phase II ALTITUDE study dose levels 1 and 2: One year results. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day. November 3, 2023; San Francisco. Boyer DS. Suprachoroidal delivery of ABBV-RGX-314 for neovascular AMD: Results from the Phase II AAVIATE study. Paper presented at the American Society of Retina Specialists 41st annual scientific meeting. July 30, 2023; Seattle, WA. Fischer MD. Pooled safety analysis of a single intravitreal injection of JNJ-1887 (Gene Therapy, AAVCAGsCD59) in patients with age-related macular degeneration (AMD). Paper presented at EURETINA 2023. October 7, 2023; Amsterdam. |
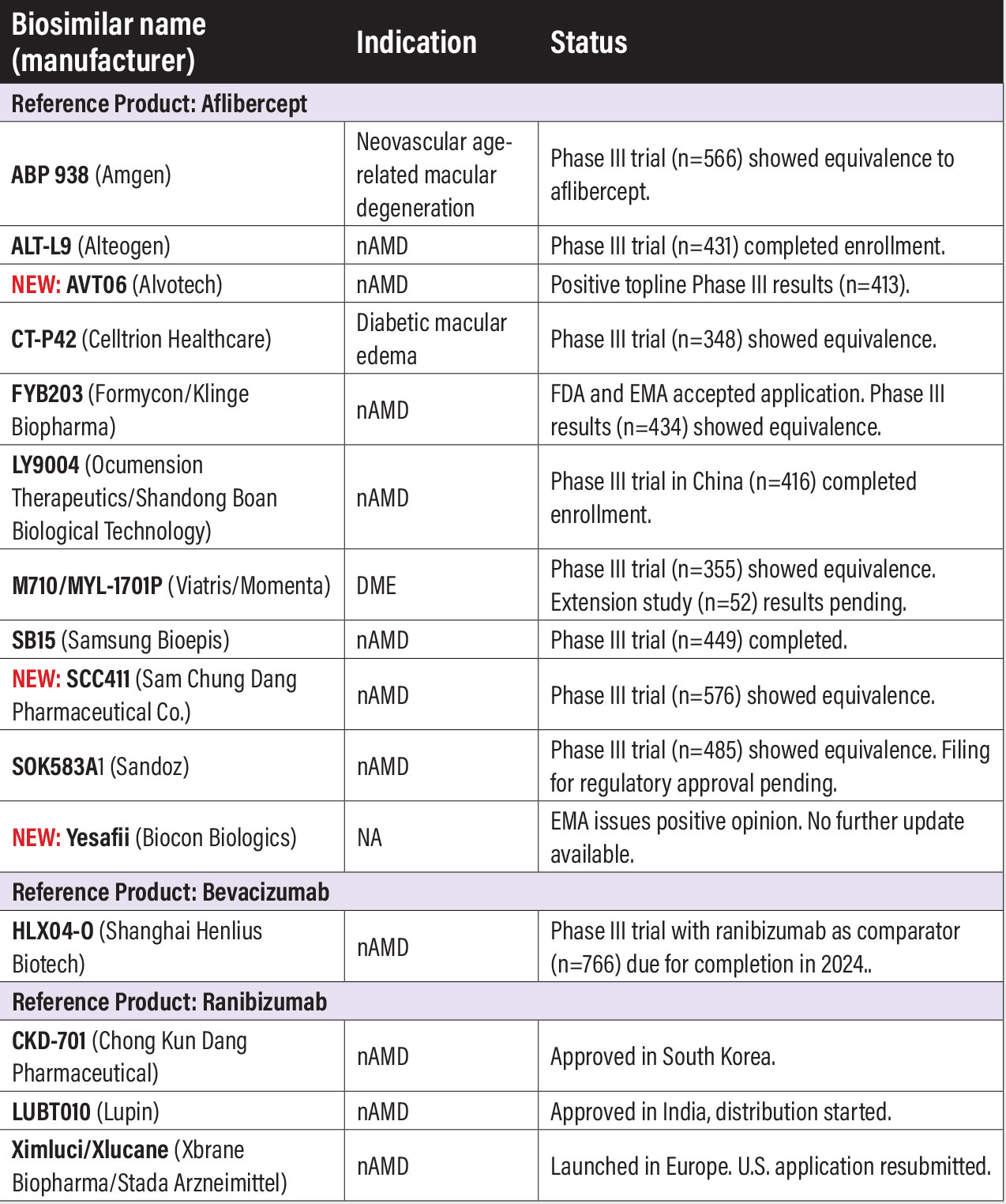
Anti-VEGF biosimilars in clinical trials ABP 938 (Amgen). The U.S. Food and Drug Administration accepted the biologics license application (BLA) for ABP 938. Final analysis from a Phase III study in neovascular age-related macular degeneration (n=566, NCT04270747) confirmed no clinically meaningful differences in efficacy between the biosimilar and reference product. A separate Phase III trial (n=49, NCT05704725) is evaluating ABP 938 in chorioretinal vascular disease. ALT-L9 (Alteogen). Alteogen last year completed enrollment in the Phase III comparator trial with aflibercept in nAMD (n=431, EudraCT: 2021-004530-11). NEW: AVT06 (Alvotech). This Iceland-based company reported positive top-line results from a Phase III trial of AVT06 in nAMD (n=413, NCT05155293). CT-P42 (Celltrion). Twenty-four week results from the Phase III trial in diabetic macular edema (n=348, NCT04739306) demonstrated equivalence to aflibercept for eight-week improvement in best-corrected visual acuity. Celltrion said last year it plans to complete the Phase III trial and file for licensure of CT-P42 in the United States and Europe, but no further update was available at press time. FYB203 (Formycon/Klinge Biopharma). The FDA and European Medicines Agency (EMA) last year accepted the development partners’ application. Results from the Phase III MAGELLAN-AMD trial in nAMD (n=434, NCT04522167) demonstrated equivalency with aflibercept. LY9004 (Ocumension Therapeutics/Shandong Boan Biological Technology). China-based Ocumension reported last year that the Phase III clinical trial completed enrollment (n=416). The trial isn’t registered at ClinicalTrials.gov or the European Union Clinical Trials Register (EudraCT). Boan holds licensing rights outside China. M710/MYL-1701P (Viatris/Mylan Pharmaceuticals). Results from the Phase III trial in central DME (n=355, NCT03610646) demonstrated comparable vision and anatomical outcomes with the reference product. Eligible subjects from the study would be enrolled in the AFIL-IJZ-3002 extension study (n=52, NCT04674800). Results are still pending from the extension study. SB15 (Samsung Bioepis). A post-hoc analysis of data from a Phase III trial in patients with nAMD (n=449, NCT04450329) demonstrated those who switched from aflibercept to SB15 maintained comparable clinical efficacy and safety.1 NEW: SCD411 (Sam Chun Dang Pharmaceutical Co.). Sam Chun Dang last year completed a Phase III trial in nAMD (n=576, NCT04480463) that demonstrated equivalence with aflibercept in visual and safety outcomes. SOK583A1 (Sandoz/Novartis). Sandoz, a division of Novartis, reported comparable efficacy and safety results between the biosimilar and reference product from the Phase III MYLIGHT trial (n=485, NCT04864834) in nAMD. Sandoz said last year it would file for regulatory approval for biosimilar aflibercept in the United States and European Union “in the coming months.” NEW: Yesafili (Biocon Biologics). The EMA last July issued a positive opinion recommending approval of Yesafili, but no update has been issued since.
HLX04-O (Essex Bio-Technology/Shanghai Henlius Biotech). U.S. patients started enrollment in the Phase III trial in nAMD that had already dosed patients in the European Union and Australia (n=388, NCT04740671), and the first patients were dosed in a Phase III trial in China (n=388, NCT05003245). Both trials are using ranibizumab, not the reference product bevacizumab, as the comparator. Both trials are set for completion this year. Results from a Phase I/II single-arm study in nAMD (n=20, NCT049933352), completed last year, demonstrated acceptable safety and tolerability in treated patients. The biosimilar is already approved in China for cancer indications.
CKD-701 (Chong Kun Dang Pharmaceutical). South Korea last year approved CKD-701. A Phase III trial in nAMD with ranibizumab as the comparator in 2022 found the biosimilar met predefined equivalence criteria (n=312, NCT04857177). The trial was conducted in South Korea. Chong Kun Dang has a host of affiliations with U.S. companies, including Pfizer and Amgen. LUBT010 (Lupin). A Phase III comparator trial with the reference product in nAMD in India is now listed as “unknown status” (n=600, NCT04690556). However, Lupin did receive approval in India for LUBT010 for all indications the reference product is approved for. Lupin is distributing LUBT010 in the Middle East. Ximluci/Xlucane (Xbrane Biopharma/STADA Arzneimittel). Ximluci launched last year in Europe. In 2022 STADA withdrew the BLA it filed with the FDA, but resubmitted it last year and the FDA accepted it. The Biosimilar User Fee Amendment goal date is set for April. A Phase III in nAMD (n=582, NCT03805100), completed in 2022, demonstrated equivalency with the reference product. Bausch + Lomb has an agreement with Xbrane and STADA to commercialize Ximluci in the United States and Canada.
REFERENCE Sadda S, Woo SJ, Bradvica, et al. Pre-to-post switching efficacy and safety assessments of SB15 (proposed aflibercept biosimilar) in neovascular age-related macular degeneration: Findings from a post-hoc analysis of a Phase III clinical trial. Paper presented at EURETINA 2023. October 7, 2023; Amsterdam. |
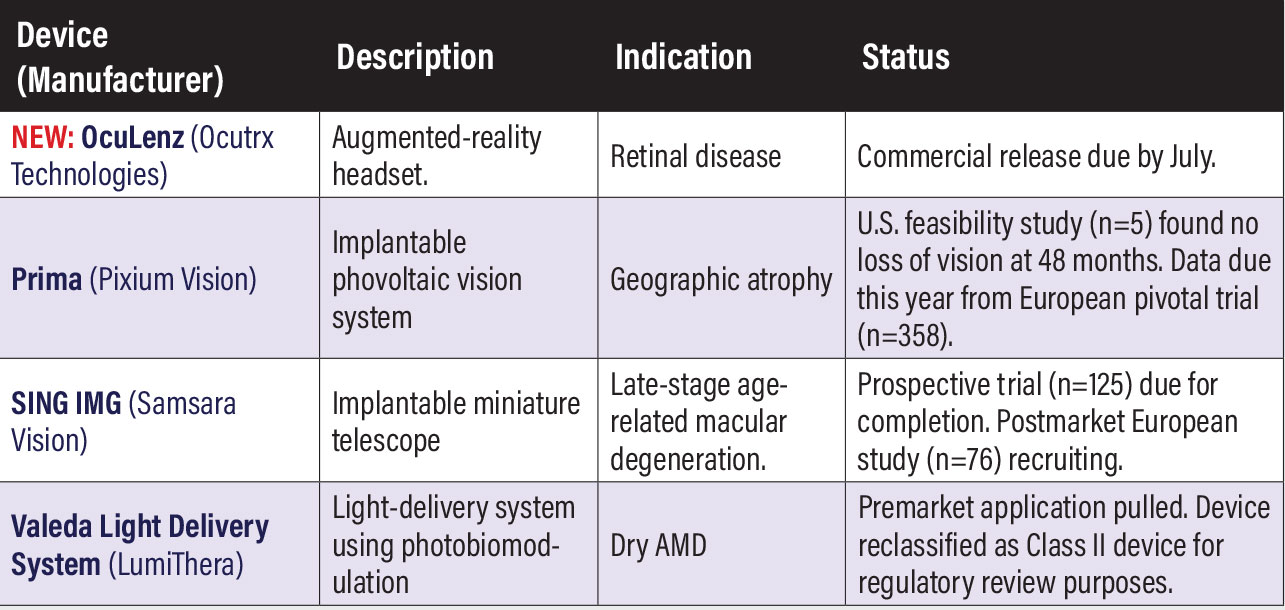
Investigative device-based therapies Prima (Pixium Vision). This intraocular implant consists of a photovoltaic subretinal prosthesis for patients with vision loss from atrophic dry age-related macular degeneration. The first in-human study (n=5, NCT03392324) demonstrated the feasibility of implanting the device, with no participants reporting reduction of natural peripheral visual function after 48 months. Pixium says it expects to report full data this year from the PRIMAvera trial (n=38, NCT04676854), its European pivotal study. SING IMT (Samsara Vision). It stands for smaller-incision, new-generation, implantable miniature telescope, a 10.8-mm telescope implanted in the eye through a small incision similar to cataract surgery. The prospective CONCERTO study (n=125, NCT05438732) of older adults living with stable, bilateral central scotomas due to late-stage AMD and fovea-involving geographic atrophy or disciform scar is set for completion this year. A postmarket European study in late-stage AMD (n=76, NCT04796545) is currently recruiting. Valeda Light Delivery System (LumiThera). Valeda is a photobiomodulation (PBM) treatment platform. LumiThera has filed a de novo request with the U.S. Food and Drug Administration to reclassify Valeda as a Class II device. LumiThera submitted to the FDA data from the LIGHTSITE III trial in dry AMD (n=96, NCT04065490), which showed statistically significant improvement in best-corrected visual acuity at 13 months in the PBM group vs. sham. The data were part of the FDA premarket approval application filed last year. However, after the FDA reviewed the application, LumiThera determined the best path to market would be the de novo route to reclassify the device as a Class II device, which shortens the review time to 150 vs. 180 days. LumiThera last year launched a clinical registry study, EUROLIGHT, to evaluate outcomes on up to 1,000 patients who had previous PBM therapy.
|
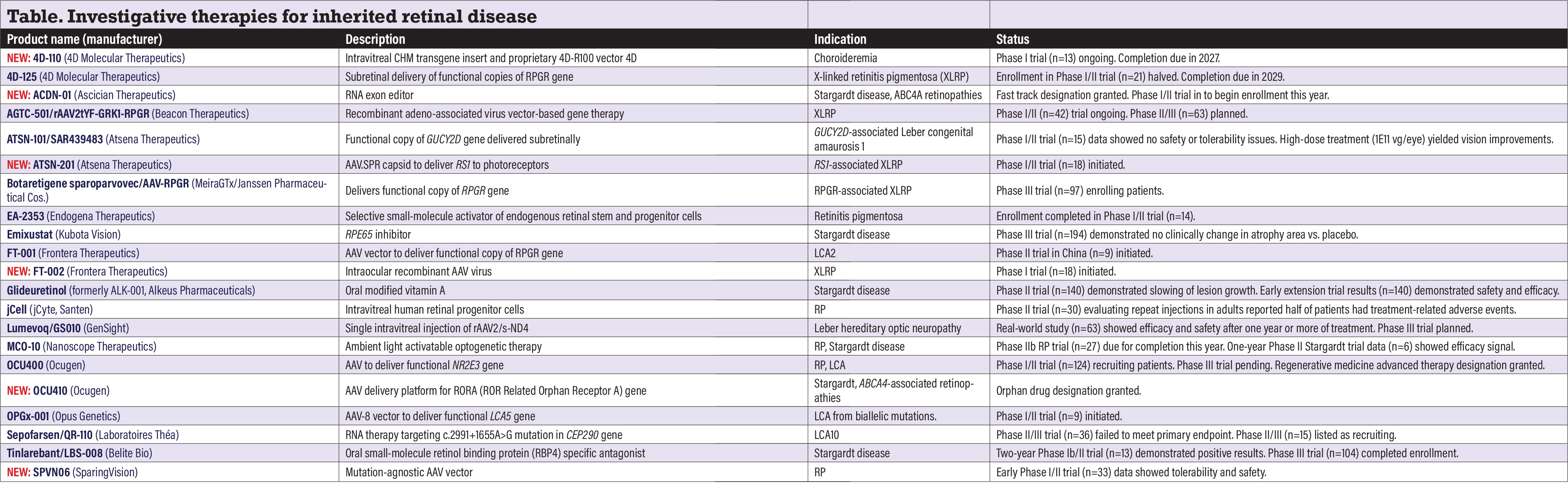
Investigative therapies for inherited retinal disease
NEW: 4D-110 (4D Molecular Therapeutics)
4D-110 is a CHM transgene insert using the proprietary vector 4D-R100, which is designed to provide targeted delivery via intravitreal administration and to transduce all retinal layers. The dose-escalation Phase I CHORUS study (n=13, NCT04483440) in patients with choroideremia is ongoing, with completion estimated in 2027.
4D-125 (4D Molecular Therapeutics)
4D-125 is designed to deliver a functional copy of the retinitis pigmentosa GTPase regulator (RPGR) gene to retina photoreceptors. The Phase I/II
EXCEL study (n=21, NCT04517149) in x-linked retinitis pigmentosa (XLRP) completed enrollment, but the enrollment target is half of what it was last year. Full study completion is anticipated in 2029.
NEW: ACDN-01 (Ascidian Therapeutics)
Ascidian describes this as the “first-ever” clinical-stage RNA exon editor and the only therapy targeting the genetic cause of Stargardt disease. The U.S. Food and Drug Administration approved an investigational new drug (IND) application and granted it fast track designation. Ascadian says it expects to open enrollment in a Phase I/II trial in Stargardt and other ABCA4 retinopathies in the first half of the year.
AGTC-501/rAAV2tYF-GRK1-RPGR (Beacon Therapeutics)
This recombinant adeno-associated viral (AAV) vector-based therapy targets mutations in the RPGR gene. The Phase I/II SKYLINE trial (n=42, NCT03316560) is continuing to recruit patients, with completion set for 2026. A Phase II/III VISTA trial evaluating the vector is still planned but isn’t yet recruiting (n=63, NCTO4850118). Syncona last year acquired Applied Genetic Technologies Corp. and folded it into Beacon Therapeutics.
ATSN-101 (formerly SAR439483, Atsena Therapeutics)
ATSN-101is a subretinally administered AAV-based therapy for Leber congenital amaurosis caused by biallelic mutations in the GUCY2D gene. Twelve-month data from the Phase I/II trial (n=15, NCT03920007) found no serious treatment-emergent adverse events.
In high-dose patients, the mean change from baseline in dark-adapted full-field stimulus testing (white stimulus) was greater than in untreated eyes. Some patients exhibited more than 10,000-fold improvements in retinal sensitivity. Study completion is due in 2027. The FDA early this year granted ATSN-101 rare pediatric disease designation.
NEW: ATSN-201 (Atsena Therapeutics)
As Atsena explains it, ATSN-201 “leverages” the novel spreading capsid AAV.SPR, facilitating delivery of RS1 to photoreceptors in the fovea. Preclinical studies demonstrated that AAV.SPR promotes transgene expression beyond the subretinal injection bleb margins.
The company last year opened recruitment in the Phase I/II LIGHTHOUSE trial (n=18, NCT05878860) in RS1-associated XLRP. Completion is set for 2029.
Botaretigene sparoparvovec/AAV-RPGR (MeiraGTx Holdings/Janssen Pharmaceutical Cos.)
Through a one-time administration, the vector otherwise known as bota-vec aims to deliver functional copies of the RPGR gene that may counteract the loss of retinal cells in RPGR-associated XLRP. The Phase III LUMEOS trial (n=97, NCT04671433) is still recruiting.
Bota-vec has been granted FDA fast track and orphan drug designations. Completion of the LUMEOS trial is anticipated for 2025.
EA-2353 (Endogena Therapeutics)
This is a small molecule agent that selectively activates endogenous retinal stem and progenitor cells that differentiate into photoreceptors. The company completed enrollment last year in the Phase I/IIa trial in retinitis pigmentosa (n=14, NCT05392751). Study completion is set for 2025.
Emixustat (Kubota Vision)
Emixustat is a once-daily oral tablet that aims to inhibit RPE protein 65 (RPE65). Two-year results from the Phase III trial in Stargardt disease (n=194, NCT03772665) demonstrated no clinically significant change in total area of macular atrophy measured with fundus autofluorescence between the treated and placebo arms. Kubota still lists the program on its website.
FT-001 (Frontera Therapeutics)
FT-001 is an AAV gene therapy administered by a one-time subretinal injection that aims to deliver a functional copy of the RPE65 gene. Frontera last year initiated a Phase II trial (n=9, NCT05858983) in patients with biallelic RPE65 mutation-associated retinal dystrophy. The study is being conducted in China.
NEW: FT-002 (Frontera Therapeutics)
FT-002 is an intraocular injection of recombinant AAV virus carrying the gene that expresses active functional proteins and repairs damaged retinal cells. Frontera last year dosed the first patient in a Phase I XLRP trial (n=18, NCT05874310). Completion is set for 2027.
Glideuretinol (formerly ALK-001, Alkeus Pharmaceuticals)
Glideuretinol is an oral modified form of vitamin A designed to significantly reduce the propensity of vitamin A dimerization, the process by which molecules bind together to form a dimer, and build up byproducts and lipofuscin in the retina. Results from the Phase II TEASE-1 trial in Stargardt disease (n=140, NCT02402660) demonstrated glideuretinal slowed the growth of atrophic lesions 21 to 28 percent.1 Completion is scheduled for 2025.
Alkeus reported early results from an open-label extension trial known as TEASE-3 (n=140, NCT 04239625) in early stage Stargardt demonstrated the first three teenage patients were asymptomatic and free of disease progression for two to six years.
jCell (jCyte, Santen)
jCell is an intravitreal injection of human retinal progenitor (hRPC) cells that aims to preserve or potentially restore some vision in RP and related conditions. A Phase II trial evaluating the safety of repeat injections in adults with RP (n=30, NCT04604899) posted results last year. This study used the 6 x 106 vector genes per eye (vg/eye) dose. Half of patients had some type of treatment-related adverse event, two of which (6.7 percent) were serious.
Lumevoq/GS010 (GenSight Biologics)
Lenadogene nolparvovec is another name for this candidate, a single intravitreal injection of rAAV2/2-ND4 for patients with Leber congenital optic neuropathy due to a mutated ND4 mitochondrial gene. A real-world study last year (n=63) confirmed efficacy and safety in patients a year or more after the injection. Mean BCVA in all eyes increased on average by 22.5 Early Treatment Diabetic Retinopathy Study letters.2
The European Medicines Agency last year signed off on a new Phase III control-vs.-sham trial, called RECOVER. GenSight says it will also engage with the FDA regarding RECOVER. The trial hasn’t yet been posted on ClnicalTrials.gov. Results from the Phase III RESCUE trial (n=39, NCT02652767), posted in 2022, showed sustained VA improvement in treated patients.
MCO-010 (Nanoscope Therapeutics)
The MCO platform is designed to deliver light-sensitive multicharacteristic opsins into retina cells. Nanoscope is due this year to complete the Phase IIb RESTORE trial in RP (n=27, NCT04945772). One-year data from the STARLIGHT Phase II trial in Stargardt disease (n=6, NCT05417126) reported that two of three patients with the macular phenotype had a 3-line BCVA improvement, and that the treatment was well-tolerated without any serious adverse events.3
Two observational, long-term follow-up studies were initiated last year, both enrolling by invitation only: in RP (n=18, NCT06162585); and Stargardt disease (n=6, NCT048185).
OCU400/AAV-NR2E3 (Ocugen)
OCU400 is a modified gene therapy that targets nuclear hormone receptors (NRH) for RP associated with mutations in the Nr2e3 gene and rhodopsin, and LCA with mutations in the CEP290 gene. The FDA granted regenerative medicine advanced therapy designation for the RP indication. Ocugen also says it “received alignment” from the FDA on key pieces of the Phase III trial design. A Phase I/II trial for both indications is recruiting (n=124, NCT05203939).
Ocugen obtained FDA approval last year to enroll pediatric patients in the trial. Early trial results demonstrated a favorable safety and tolerability profile so far, the company reports. Enrollment is expected to conclude this year.
NEW: OCU410 (Ocugen)
OCU410 uses an AAV delivery platform for the retinal delivery of the RORA (ROR Related Orphan Receptor A) gene. The FDA last year granted orphan drug designation for treatment of ABCA4-associated retinopathies, including Stargardt disease, RP 19 and cone-rod dystrophy 3 (CORD3), but the trial isn’t registered yet. OCU410 is also the focus of a clinical trial in dry AMD.
OPGx-001/OPGx-LCA5 (Opus Genetics)
OPGx-001 is a subretinal AAV-8 vector designed to precisely deliver a functional LCA5 gene to retinal photoreceptors. The first patient was dosed in a Phase I/II trial in patients with LCA resulting from biallelic mutations in the LCA5 gene (n=9, NCT05616793). The trial is due for completion in 2027.
Sepofarsen/QR-110 (Laboratoires Théa)
This RNA therapy is indicated for LCA10 due to the c.2991+1655A>G mutation in the CEP290 gene. Théa last year acquired the rights from ProQR, canceled the transaction, then came back and closed the deal in December. Théa says it will continue development.
ProQR reported in 2022 that the Phase II/III ILLUMINATE trial (n=36, NCT03913143) failed to meet its primary endpoint of improved best-corrected visual acuity. The Phase II/III BRIGHTEN trial in children with LCA10 caused by mutations in the CEP290 gene (n=15, NCT04855045) is still listed as recruiting, but no update has been posted since March 2022.
Tinlarebant/LBS-008 (Belite Bio)
Tinlarebant is an oral therapy that aims to reduce the accumulation of vitamin A-based toxins, known as bisretinoids, that contribute to retinal pathology in Stargardt disease. Two-year results from the Phase Ib/II study in adolescent patients with STGD1 (n=13, NCT05266014) demonstrated sustained lower decreased autofluorescence lesion growth as well as stabilized VA in a majority of patients.4 Belite Bio last year completed recruitment in the Phase III DRAGON trial (n=104, NCT05244304) in STGD1. Completion is anticipated in 2025.
NEW: SPVN06 (SparingVision)
SparingVision describes SPVN06 as a mutation-agnostic, AAV gene therapy composed of one neurotrophic factor (rod-derived cone viability factor, RdCVF) and one oxidative stress-reducing enzyme (RdCVF long form), designed to act together to slow or stop the degeneration of photoreceptors.
Initial safety data from the first three-patient cohort treated in the Phase I/II PRODYGY trial in RP (n=33, NCT05748873) demonstrated a favorable safety profile, SparingVision reports. The trial is scheduled for completion in 2029. SparingVision is also developing a preclinical candidate for RP, SPVN20. RS
 |
| Click image to view table in the digital edition. |
REFERENCES
Kay C. ALK-001 (C20-D3-Vitamin A) slows the growth of atrophic lesions in ABCA4-related Stargardt disease: results of a randomized, placebo-controlled clinical trial, the TEASE study. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day. November 3, 2023; San Francisco, CA.
Vignal-Clermont C. Use of lenadogene nolparvovec gene therapy for Leber Hereditary optic neuropathy in early access programs. Paper presented at North American Neuro-Ophthalmology Society. March 14, 2023; Orlando, FL.
Ho A. MCO-010 optogenetic therapy for profound vision loss in Stargardt disease: 12-month outcomes from the Phase 2 STARLIGHT trial. Paper presented at Retina Society 56th annual scientific meeting. October 12, 2023: New York, NY.
Nguyen QD. Tilarebant (LBS-008) in adolescent subjects with Stargard disease. Paper presented at American Academy of Ophthalmology Retina Subspecialty Day. November 4, 2023; San Francisco, CA.