Take-home points
|
 |
Bios Dr. Khalid is a junior doctor, MBBS triple merit, iBSC honors at Machester Royal Infirmary, Manchester, U.K. Dr. Dhoot practices at California Retina Consultants in Santa Barbara, and is an adjunct clinical assistant professor of ophthalmology at USC’s Keck School of Medicine. Disclosures: Dr. Khalid has no financial interests to disclose. Dr. Dhoot has the following financial interests: Consultant: Apellis Pharmaceuticals, Alimera, Allergan, Bayer, Biocryst, Coherus, EyePoint, Genentech, Iveric Bio, Ocular Therapeutix, Outlook Therapeutics, Oxular, Regeneron, RegenXBio, Roche, Novartis Stockholder: Outlook Therapeutics and Vortex Surgical. |
Vascular endothelial growth factor has been implicated in the pathogenesis of a range of conditions, including neovascular age-related macular degeneration and diabetic macular edema.1 As we all know, the use of anti-VEGF therapy has dramatically revolutionized the treatment of these conditions, leading to both visual and anatomic benefits.2
First approved in 2006, ranibizumab is an anti-VEGF monoclonal antibody fragment indicated for the treatment of neovascular age-related macular degeneration among other VEGF-driven eye conditions.3 Anti-VEGF agents like ranibizumab have traditionally been delivered via frequent intravitreal injections as often as every four weeks. This approach results in a significant burden to patients and their caregivers. Moreover, each injection carries risk, the most feared being endophthalmitis, which risks potential sight-threatening consequences.
A recognized unmet need in the treatment of NVAMD is durability. More durable treatments have the potential to reduce cumulative long-term risk as well as burden for patients. The biologic agents currently used typically have short intraocular half-lives, thus the development of a long-acting ocular drug delivery platforms is ideal for increasing treatment intervals.4
Here, weʼll discuss one of the options to help ease this treatment burden, the Port Delivery System with Ranibizumab, and share pearls for its use in anticipation of its potential re-introduction into the U.S. market.
The Port Delivery System
The PDS was first approved by the United States Food and Drug Administration in October of 2021 for NVAMD in adults who have previously responded to at least two anti-VEGF injections.3 The PDS allows for continuous delivery of ranibizumab and requires two refills a year thereby reducing the burden associated with frequent injections.
The PDS is surgically implanted at the pars plana and allows for sustained delivery of a customized drug formulation of ranibizumab (Figure 1). The ocular implant is refilled using a proprietary refill needle via an in-office refill-exchange procedure.
The Phase III Archway trial evaluated the safety and efficacy of PDS with Ranibizumab with 24-week refill exchanges versus monthly intravitreal anti-VEGF injections for treatment of neovascular AMD in patients responsive to anti-VEGF therapy.5 The study demonstrated noninferiority and equivalence of the PDS in achieving vision outcomes in comparison with intravitreal ranibizumab injections.6,7
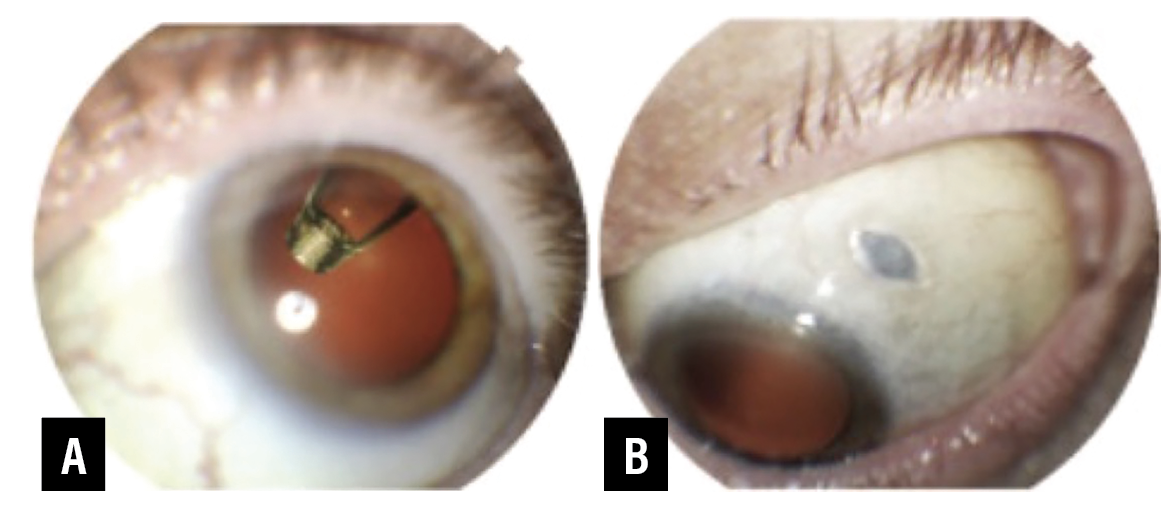 |
|
Figure 1. Port Delivery System with Ranibizumab. A) Supero-temporal gaze with implant visible through dilated pupil. B) Inferonasal gaze allowing visualization of PDS septum.5 (All images: Copyright 2021 F. Hoffmann-La Roche, all rights reserved. Used with permission.) |
Voluntary recall/potential relaunch
In October 2022, Genentech/Roche initiated a voluntary recall of the PDS ocular implant and insertion tool assembly after identifying that the implant didn’t meet pre-specified standards. Refill-exchange procedures could continue in patients who had already received an implant, however.8 A thorough root-cause investigation was conducted. Updates to the PDS implant and the refill needle have been implemented to optimize performance and mitigate the risk of septum dislodgment which has resulted in the resumption of PDS clinical studies and an anticipated relaunch in the commercial setting.
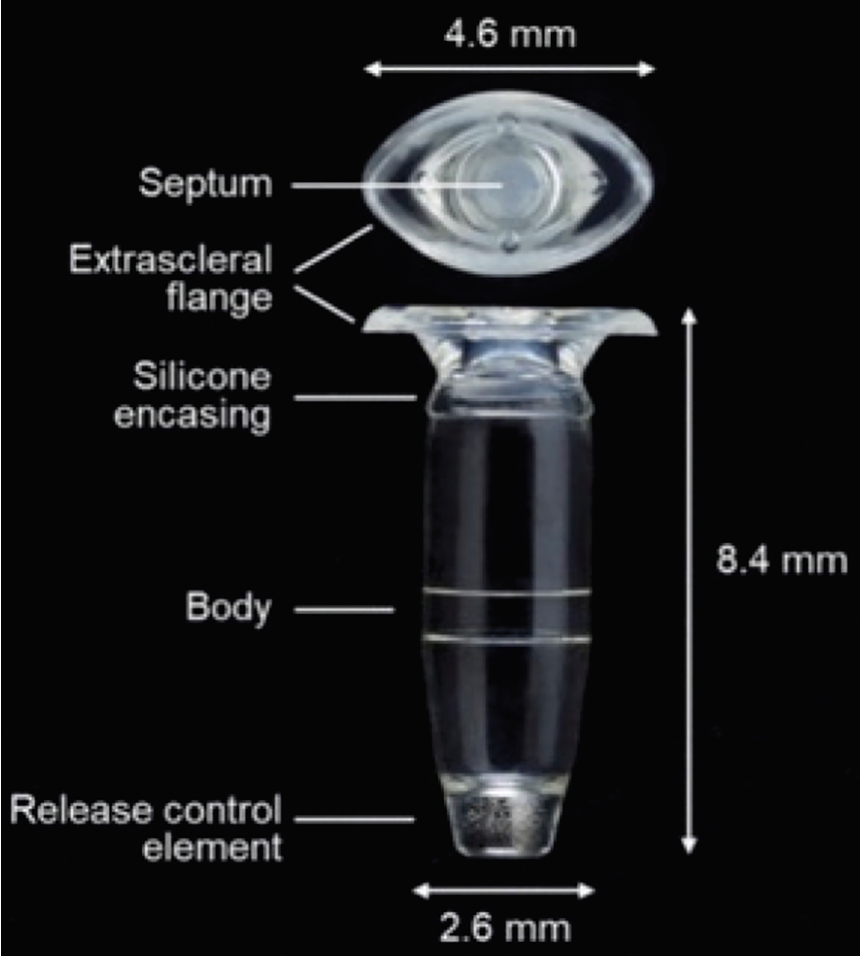 |
| Figure 2. Components of the Port Delivery System with Ranibizumab.9 |
Refill-exchange
Understanding the nuances of the PDS implantation and surgical technique is vital to the successful performance of the device. The nuances of the in-office refill-exchange procedure are also equally important to understand for optimal device performance. The refill-exchange is within the wheelhouse of the practicing retina specialist, though the procedure differs from a typical intravitreal injection. The individual elements of the PDS and their dimensions are illustrated in Figure 2. The implant body can hold 20 µL of drug.
The solution within the implant body is exchanged via the refill-exchange procedure wherein existing ranibizumab is replaced with fresh drug. An injection of 0.1 mL of drug can replace >95 percent of implant contents, as demonstrated in Figure 3.9
Preparing for the procedure
The refill-exchange procedure is performed under strict aseptic conditions, typically in an office-based setting.
Dilated slit-lamp examination (and/or indirect ophthalmoscopy) should be carried out prior to the procedure to inspect the implant in the vitreous cavity, through the pupil. This helps to identify if septum dislodgement has occurred, in which case no further refill-exchange procedures should be performed as normal device functioning can’t be assured.10
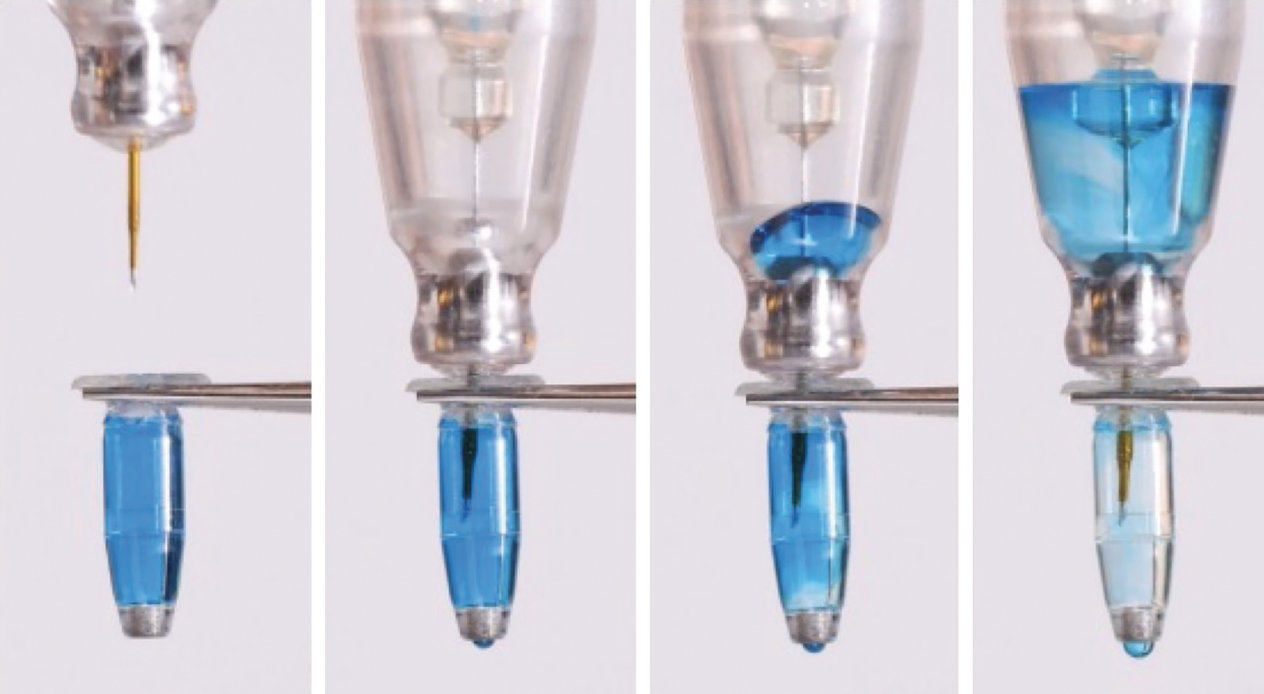 |
|
Figure 3. Demonstration of displacement of implant contents while injecting fresh new solution.9 |
A slit lamp exam should additionally be conducted to identify the clock-hour location of the implant and any landmarks which may aid in septum targeting. Careful examination of the conjunctiva should be performed to exclude conjunctival retraction or erosion, implant exposure or other complications.
Following this, we recommend the Environment, Visualization, Perpendicularity (EVP) strategy which has been developed through clinical experience for a successful refill-exchange procedure.11 EVP is explained further below.
Setting up for a successful refill-exchange procedure: EVP
The following is key to understanding the EVP concept:
• Environment. Creating an optimal environment helps to ensure a smooth procedure. The key steps for this can be remembered by the acronym SEPTM (Sterility, Eyelid Speculum, Positioning, Task lighting and Magnification).
Sterility is paramount for avoiding infectious complications; the procedure should be conducted within a sterile field, with a surgical mask and sterile gloves worn throughout (Figure 4). Needles and syringes should be transferred into the field as required. A broad-spectrum microbicide such as povidone-iodine should be applied to the periocular skin, eyelid and ocular surface. An optional sterile drape may be used, and a topical antimicrobial applied to the fornix.10
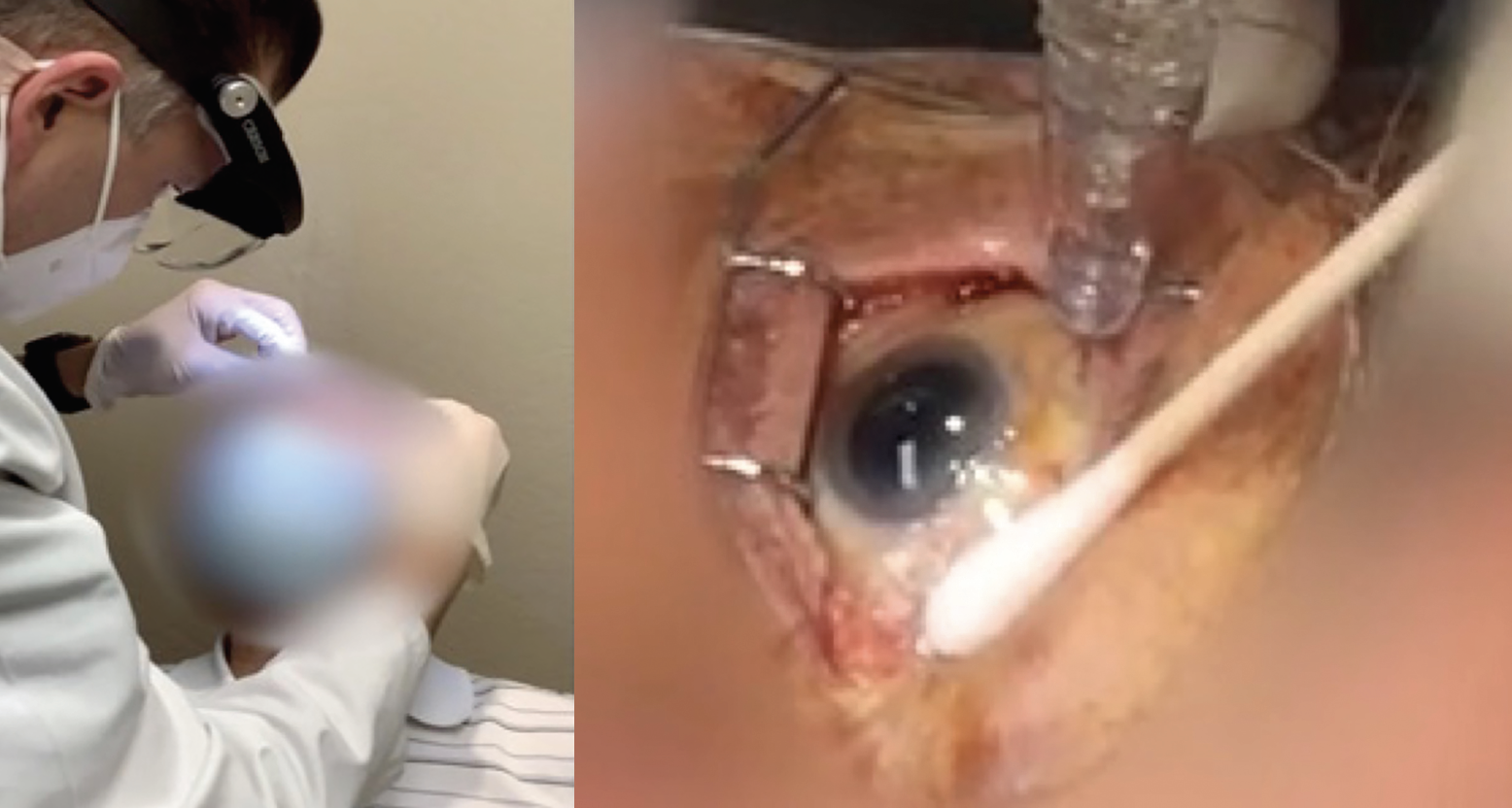 |
Figure 4. The procedure is performed in a sterile in-office environment.9 |
The patient is optimally positioned supine at a 20 to 30 degree angle with the chin up. Use of an eyelid speculum facilitates easy access for the physician and frees up their hands for precise needle positioning.
The physician should consider standing on the contralateral side to the implanted eye. This allows the physician to target the center of the implant septum while maintaining a perpendicular direction of entry of the refill needle. It additionally allows good visualization of the septum entrance point.
Lastly, optimal task lighting and magnification assist in providing a comfortable working environment for the physician.
• Visualization. The implant flange is best visualized by asking the patient to maintain an inferonasal gaze, as demonstrated in Figure 1B.
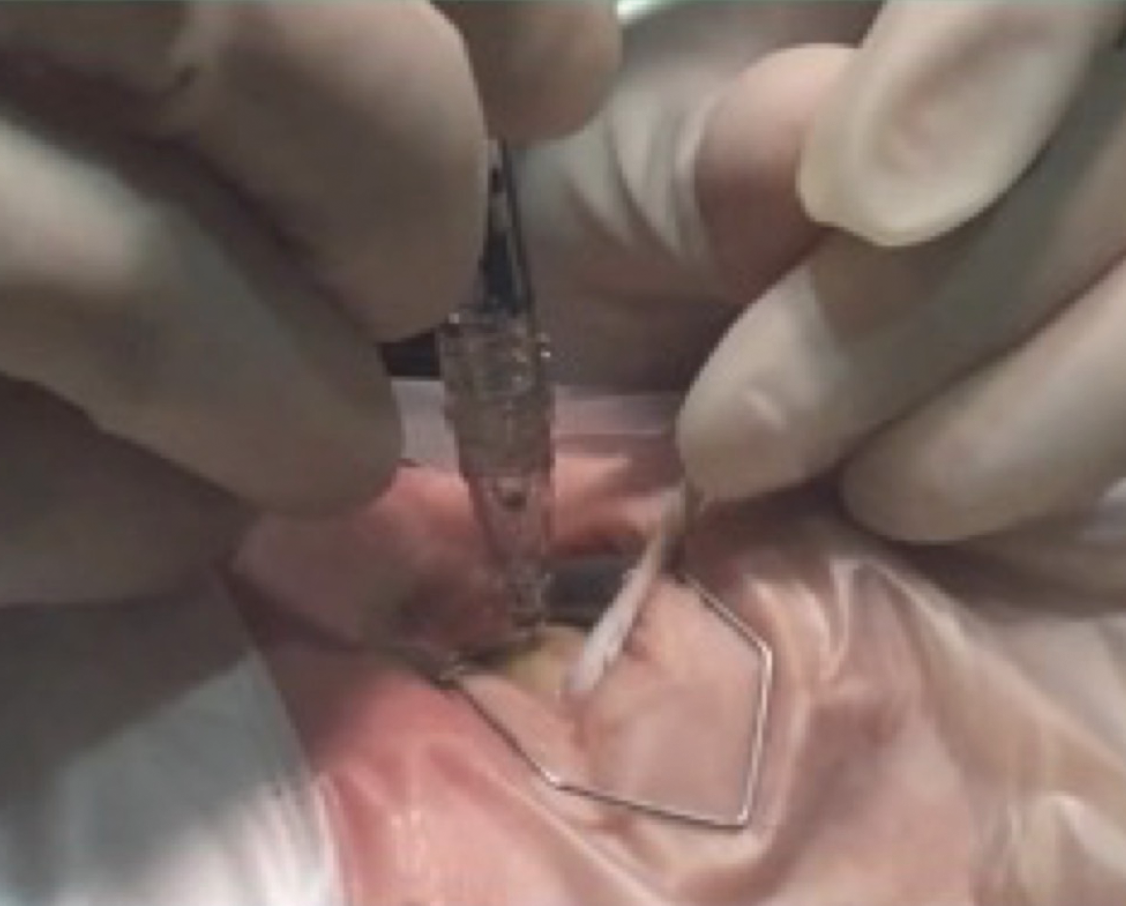
Use of a cotton-tipped applicator can be helpful to stabilize the globe, keeping the eye in the correct position for the flange to be appropriately visualized throughout the procedure. This is illustrated in Figure 5.
If visualization of the septum is proving difficult, the extrascleral flange outline may be helpful in guiding towards the expected location; the septum should be found at the intersection of the long and short axes.10
If septum visualization is challenging despite the above measures, transillumination through a dilated pupil may be helpful.
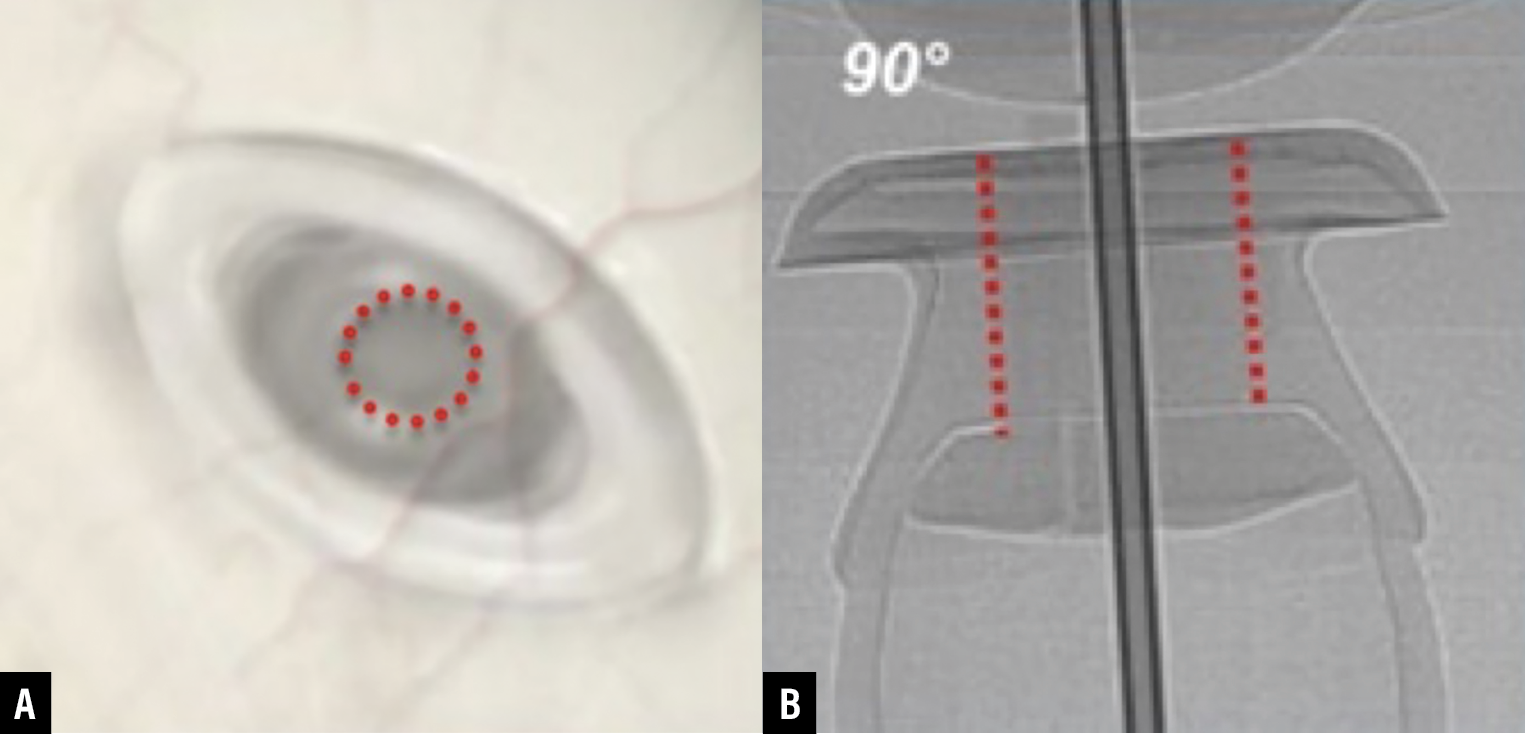 |
| Figure 6. (A) Targeting the center of the septum. (B) Perpendicular approach to entry.9 |
• Perpendicularity. The prepared syringe and refill needle should be advanced through the overlying conjunctiva and Tenon’s capsule into the septum. The septum is self-sealing, enabling access to the implant body while preventing drug egression.
The refill needle should be inserted at a 90-degree angle to the implant flange, through the Tenon’s capsule into the center of the septum (Figure 6).
 |
| Figure 7. Maintain a perpendicular orientation throughout the procedure.9 |
This orientation should then be maintained throughout the procedure as this ensures successful exchange and prevents implant movement (Figure 7).10
Perpendicularity should additionally be maintained when removing the needle, which avoids damage to the septum or conjunctiva.
Pitfalls
Some key pitfalls should be avoided when carrying out the refill-exchange procedure.
Ideally, when preparing the syringe and drawing up the ranibizumab solution, an assistant should handle the non-sterile vial. Figure 8 shows a physician holding the vial with a sterile, gloved hand, which contaminates the hand, increasing infection risk.
Twisting should not be used as a mechanism to gain access to the implant septum through the conjunctiva and Tenon’s capsule. Additionally, avoid a twisting action when attempting to reorient the angle of the needle, after entry has been gained. Twisting causes unnecessary frictional damage to both the implant septum and overlying conjunctival tissue, and may lead to septum dislodgement.
Post-procedure assessment and instructions
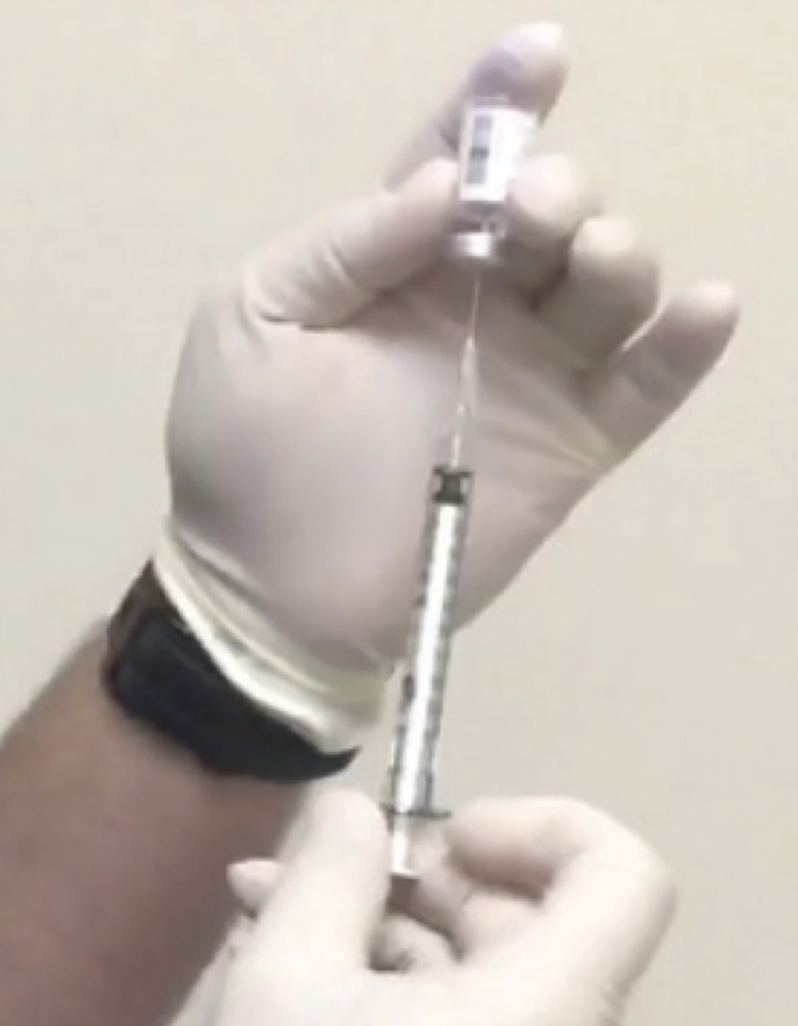 |
| Figure 8. Non-sterile vial being held with sterile gloved hand.10 |
Following the procedure, the flange of the implant should be inspected to ensure correct positioning under the conjunctiva. There should be no subluxation or displacement. The positioning of the device inside the vitreous cavity should also be inspected via dilated indirect ophthalmoscopy/slit lamp examination, at which point complications such as vitreous hemorrhage, retinal tears, retinal detachment or lens trauma should also be ruled out.
Patients should be instructed to refrain from touching or rubbing the eye, be made aware of signs and symptoms that may require immediate medical attention and to take post-procedure drops as prescribed.
The Port Delivery System with Ranibizumab results in meaningful durability gains for patients with as little as two in-office injections per year. Following a successful PDS implantation procedure, the keys to success hinge on successful and smooth in-office refill procedures.
By keeping these key pearls and pitfalls in mind, the practicing retina specialist will be positioned for success. RS
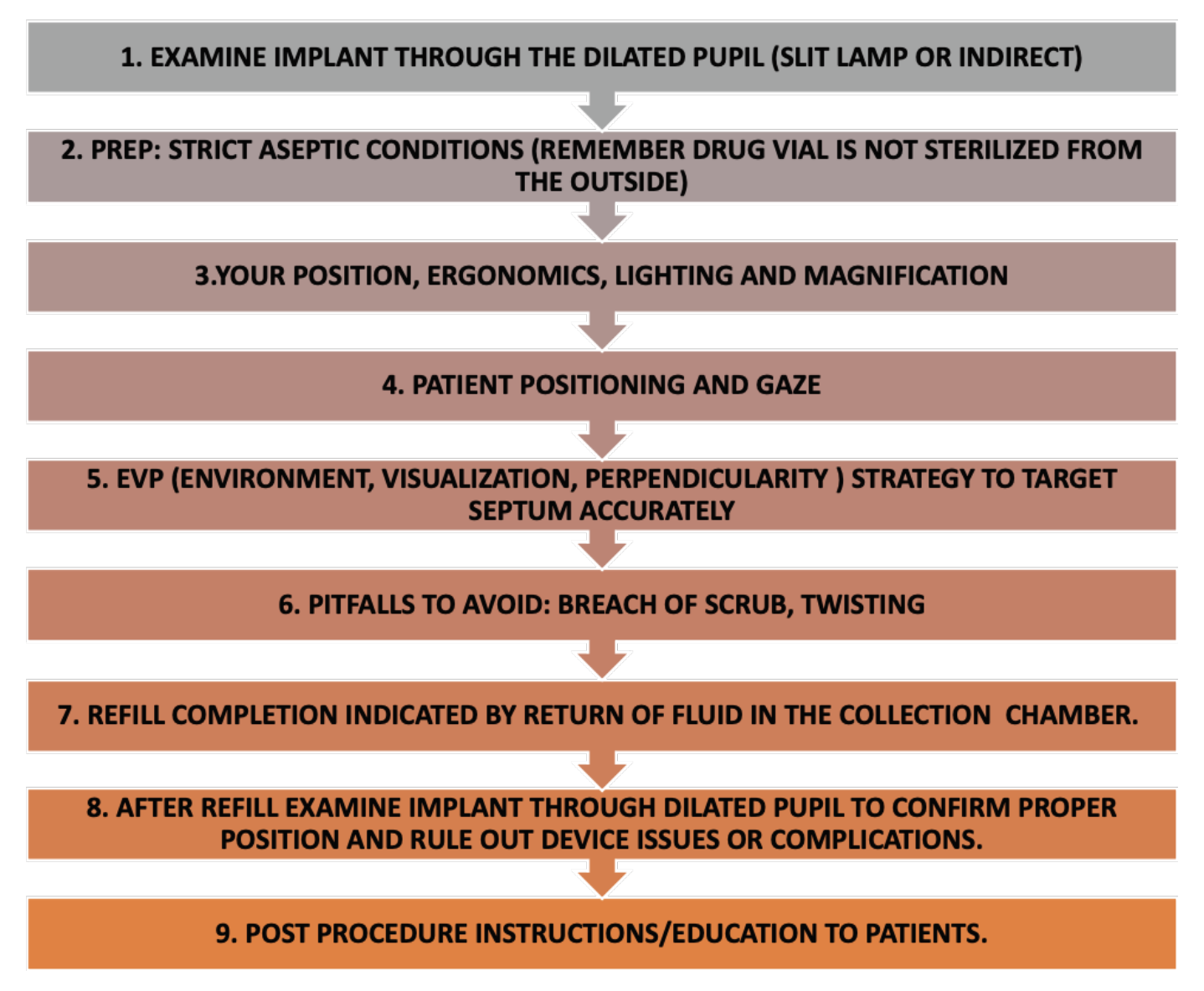 |
| A summary of key considerations while performing the refill exchange procedure. |
REFERENCES
1. Bourne RR, Jonas JB, Bron AM, Cicinelli MV, Das A, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: Magnitude, temporal trends and projections. British Journal of Ophthalmology. 2018 Mar 15;102:5:575–85.
2. Campochiaro PA. Ocular neovascularization. Journal of Molecular Medicine. 2013 18;91:3:311–21.
3. Eichenbaum DA, Ahmed A, Hiya F. Ranibizumab Port Delivery System: A clinical perspective. BMJ Open Ophthalmology. 2022 ;7:1.
4. Kim HM, Woo SJ. Ocular drug delivery to the retina: Current innovations and future perspectives. Pharmaceutics. 2021;13:1:108.
5. Ranade SV, Wieland MR, Tam T, Rea JC, Horvath J, Hieb AR, et al. The Port Delivery System with ranibizumab: A new paradigm for long-acting retinal drug delivery. Drug Delivery. 2022;29:1:1326–34.
6. Holekamp NM, Campochiaro PA, Chang MA, Miller D, Pieramici D, Adamis AP, et al. Archway randomized phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2022;129:3:295–307.
7. ClinicalTrials.gov. Roche H-L. Extension study for the Port Delivery System with ranibizumab (portal) - full text view [Internet]. U.S. National Library of Medicine; 2018. https://classic.clinicaltrials.gov/ct2/show/NCT03683251. Accessed February 22, 2024.
8. Sharma A, Khanani AM, Parachuri N, Kumar N, Bandello F, Kuppermann BD. Port Delivery System with ranibizumab (Susvimo) recall- what does it mean to the retina specialists. International Journal of Retina and Vitreous. 2023;9:1.
9. Kitchens J, Khanani AM, Vicente A, Singh N, Patel S, Jaycock P, et al. Key pearls of the refill-exchange procedure for the port delivery system with ranibizumab. Lecture presented at: 41st Annual Scientific Meeting of the American Society of Retina Specialists; United States; Jul 28,. 2023
10. Khanani AM, Graff JM, Marcus DM, Wykoff CC, Jhaveri CD, Barteselli G, et al. Refill-exchange procedure of the Port Delivery System with ranibizumab: Overview and clinical trial experience. Ophthalmic Surgery, Lasers and Imaging Retina. 2022;53:5:257–65.
11. Dhoot DS, Khanani AM, Singh N, Barteselli G, Callaway N, Patel S, et al. Key pearls of the refill-exchange procedure for the port delivery system with ranibizumab. Lecture presented at: 55th Retina Society Annual Scientific Meeting; United States; Nov 3, 2022.



