Take-home points
|
 |
Bios Dr. Guo is a vitreoretinal fellow at Stanford University School of Medicine, Palo Alto, California. Dr. Leng is an associate professor of ophthalmology and director of clinical and translational research at the Byers Eye Institute, Stanford University School of Medicine. DISCLOSURES: Dr. Guo has no relevant relationships to disclose. Dr. Leng is a consultant to Genentech/Roche. |
Diabetic macular edema is the principal cause of vision loss in individuals with diabetic retinopathy, presenting a growing public health challenge in the context of increasing diabetes prevalence. Factors like prolonged diabetes duration, inadequate blood glucose control, and persistently elevated hemoglobin A1c levels raise the risk of DME. Of course, clinical trials have shown the promise of various anti-VEGF therapies in conserving visual acuity for DME patients. Here, we delve into the real-world insights that examine the extended effectiveness of anti-VEGF agents in treating DME.
Smaller vision gains
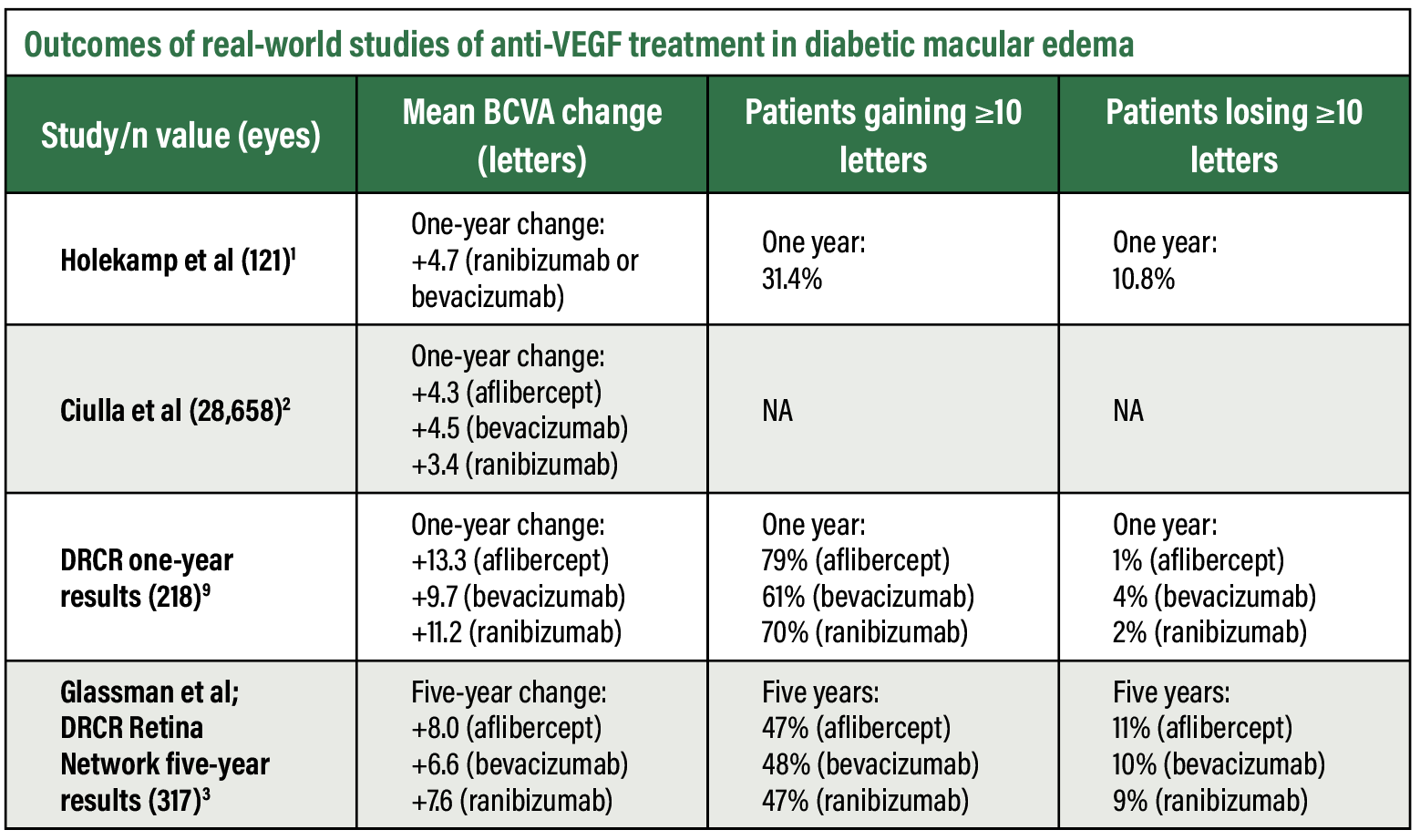
Real-world studies have shown that patients with DME (Figure 1) exhibit long-term visual acuity gains (Table below). However, the magnitude of such vision gains is much smaller compared to randomized clinical trials. The landmark DRCR Retina Network Protocol T trial showed marked visual acuity improvement with all three anti-VEGF agents— +13.3, +9.7 and +11.2 letters at one year with aflibercept, bevacizumab and ranibizumab, respectively.3 The improvement was greatest with aflibercept (Eylea, Regeneron Pharmaceuticals), with a statistically significant mean difference of 2 to 3 letters at one year.
Nancy Holekamp, MD, and colleagues examined 110 patients (121 study eyes) initiating intravitreal ranibizumab (Lucentis, Genentech/Roche) or bevacizumab (Avastin, Genentech/Roche) for DME and showed a mean corrected visual acuity change of only +4.7 letters at one year.1
In a larger retrospective study, Thomas Ciulla, MD, and colleagues examined 28,658 eyes of treatment-naive patients with DME using a database. Baseline visual acuity was 59.2 letters (20/60 Snellen equivalent) and improved by 4.2 letters at one year.2 They noted the mean one-year VA improvement was greater in patients who had more anti-VEGF injections and in patients with lower baseline VAs, with likely contribution from ceiling effects related to baseline visual acuity.
The VA improvement in this population didn’t significantly differ among the different anti-VEGF agents—+4.3, +4.5 and +3.4 letters at one year with aflibercept, bevacizumab and ranibizumab, respectively.
Real-world studies with longer follow-up showed even smaller gains in vision improvement. More recently, our group conducted an unpublished retrospective study among treatment-naive patients with DME, following treatment patterns and VA for up to six years of follow-up. At one year after anti-VEGF initiation, eyes gained a mean of +3.2 letters of vision; by six years, the mean gain was only +0.5 letters.
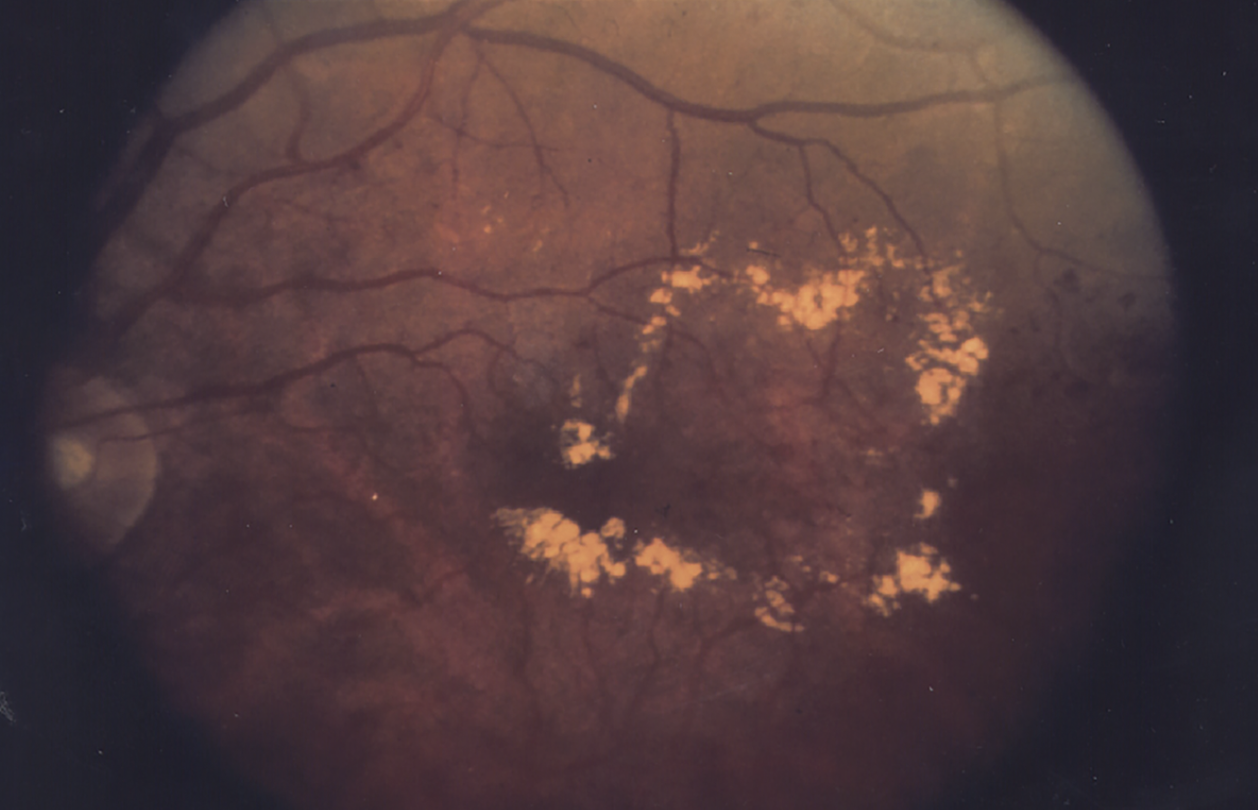 |
| Figure 1. Diabetic macular edema prior to treatment. (National Eye Institute Media Library) |
 |
Fewer injections in the real world
The DRCR Retina Network Protocol T study employed a protocol-specific algorithm with a mean of nine to 10 injections of aflibercept, bevacizumab and ranibizumab, respectively, at one year. After the clinical trial ended, the frequency of office visits and anti-VEGF injections declined.3
Multiple studies using real-world data have now shown that frequency of ophthalmology visits and injections are lower than that in clinical trials, highlighting the treatment burden of frequent intravitreal injections in patients with diabetic macular edema. In the study Dr. Ciulla led, the lower treatment intensity of six to seven injections at one year suggested that physicians are using personalized treatment plans, such as as-needed or treat-and-extend regimens, to decrease treatment burden.
This lower injection frequency likely contributes to the smaller VA improvements in the real world than in clinical trials. Our group’s study, which stratified VA outcomes by the average number of injections per year, found a +2.8 ETDRS letter improvement in those receiving one to two injections compared to +4.8 letters in those who received more than 11 injections in the first year.
This trend was consistent in subsequent years. In the long-term, patients who received five or more injections didn’t experience a best-corrected VA loss from baseline after six years of follow-up, whereas patients who received fewer than four injections a year did.
Socioeconomic disparities
Real-world data has highlighted that significant disparities in anti-VEGF use and outcomes depend on patient demographics and insurance coverage. A retrospective study using the IRIS Registry of more than 200,000 patients showed that white race, non-Hispanic/Latino ethnicity and private insurance were correlated with both higher use of anti-VEGF injections as well as higher longitudinal VA across 60 months of follow-up.4
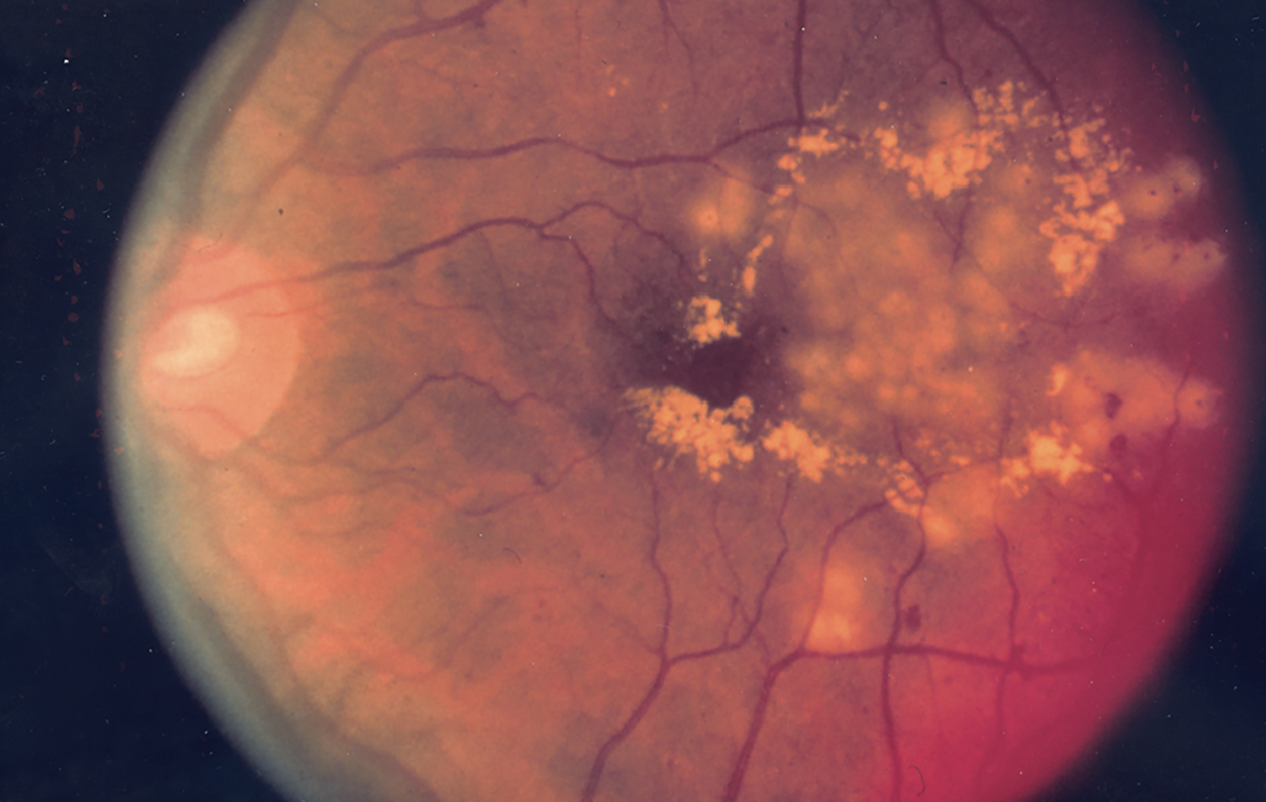 |
| Figure 2. Fundus photo showing the effect of focal laser surgery for diabetic retinopathy. (National Eye Institute Media Library) |
Multiple possible explanations exist for such disparities in outcomes. One is the potential differences in characteristics and severity of biological disease among heterogeneous populations. In a study using National Health and Nutrition Examination Survey data, the risk of diabetic retinopathy (Figure 2) was higher in Hispanic/Latino patients, even after adjusting for independent variables such as severity of diabetes (duration, hemoglobin A1c level, insulin and oral agent use) and systolic blood pressure.5
Another contributor may be access to care and adherence to clinic visits. Compared to patients with neovascular macular degeneration, patients with DME have been noted to have higher rates of nonadherence or appointment cancellation,6,7 possibly related to the presence of additional comorbidities in DME patients. A retrospective study of more than 2,000 DME patients found loss to follow-up—defined as no office visits within 12 months after an intravitreal injection—in 25.3 percent of patients.8 Factors associated with loss to follow-up included being Hispanic and Pacific Islander, and having lower adjusted gross income.8
Bottom line
Real-world treatment patterns can differ significantly from clinical trial results, since the former are influenced by both personalized treatment patterns based on provider clinical decision, as well as patient’s adherence to clinic visits and treatment. So far, real-world results have shown that the frequency of injections as well as improvement in visual acuity with anti-VEGF medications is inferior to that shown in clinical trials.
The real-world evidence highlights how the high treatment burden of diabetes significantly impacts patients’ lives. Adherence to therapy is influenced by costs, time constraints, accessibility to care and patient perception of care. Furthermore, real world evidence shows the increasingly disparate outcomes for patients in different ethnic/racial demographic groups and those with minimal or no insurance coverage. RS
REFERENCES
1. Holekamp NM, Campbell J, Almony A, et al. Vision outcomes following anti–vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83–91.
2. Ciulla TA, Pollack JS, Williams DF. Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: A real-world analysis of 28 658 patient eyes. Br J Ophthalmol. 2021;105:216–221.
3. Glassman AR, Wells JA, Josic K, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T Extension Study). Ophthalmology. 2020;127:1201–1210.
4. Greenlee TE, Malhotra NA, Iyer AI, Conti TF, Chen AX, Singh RP. Association of socioeconomic health care disparities with use of anti-vascular endothelial growth factor and visual acuity outcomes in patients with diabetic macular edema. Ophthalmic Surg Lasers Imaging Retin. 2022;53:380–391.
5. Harris MI, Klein R, Cowie C C, Rowland M, Byrd-Holt DD. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care. 1998; 21:1230–1235.
6. Ehlken C, Helms M, Böhringer D, Agostini H T, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13–20.
7. Jansen M E, Krambeer CJ, Kermany DS, et al. Appointment compliance in patients with diabetic macular edema and exudative macular degeneration. Ophthalmic Surg Lasers Imaging Retin. 2018;49:186–190.
8. Gao X, Obeid A, Aderman CM, et al. Loss to follow-up after intravitreal anti–vascular endothelial growth factor injections in patients with diabetic macular edema. Ophthalmol Retin. 2019;3:230–236.
9. Diabetic Retinopathy Clinical Research Network; Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.



