Take-home points
|
 |
Bios Dr. Rowe is an ophthalmology resident at the Glick Eye Institute, Department of Ophthalmology, Indiana University School of Medicine, Indianapolis. Dr. Ciulla is a retina specialist and board member of Midwest Eye Institute of Indianapolis, Indiana, volunteer clinical professor of ophthalmology at Indiana University School of Medicine, chief medical officer at Viridian Therapeutics, and chief medical advisor and the chair of the Scientific Advisory Board at Clearside BioMedical, where he previously served as chief medical officer and chief development officer. Disclosures: Dr. Rowe reports no relevant disclosures. Dr. Ciulla reports employment by, and holds equity in, Viridian Therapeutics. He holds equity in Clearside BioMedical and Nanoscope Therapeutics. In addition to Clearside and Nanoscope, he has served as a consultant for Ocuphire Pharma. |
Intravitreal anti-vascular endothelial growth factor injections have become the standard of care for treating neovascular age-related macular degeneration.1 Monthly or bimonthly anti-VEGF injections demonstrate favorable long-term best-corrected visual acuity preservation in real-world studies compared to less frequent dosing,2-5 but at the expense of significant time and cost burden due to the disease’s chronic nature and limited medication half-life. Gene therapy biofactories offer a potential solution to address the need for long-acting and more durable therapies in nAMD. In this article, we’ll review the latest gene-therapy efforts in this area.
Gene therapy backgrounder
Retina has been at the forefront of gene therapy in medicine, particularly since the FDA clearance of voretigene neparvovec-rzyl (Luxturna, Spark Therapeutics/Genentech) for confirmed biallelic RPE65-mediated inherited retinal disease in December 2017. Luxturna represented the first FDA-approved gene therapy for inherited disease. When a therapeutic gene is delivered and integrates into patient cells, it has the capacity to continually generate a desired protein, such as endogenous anti-VEGF, and thus offers the prospect of alleviating the treatment burden of frequent intravitreal injections for nAMD through sustained and enduring therapeutic effects.
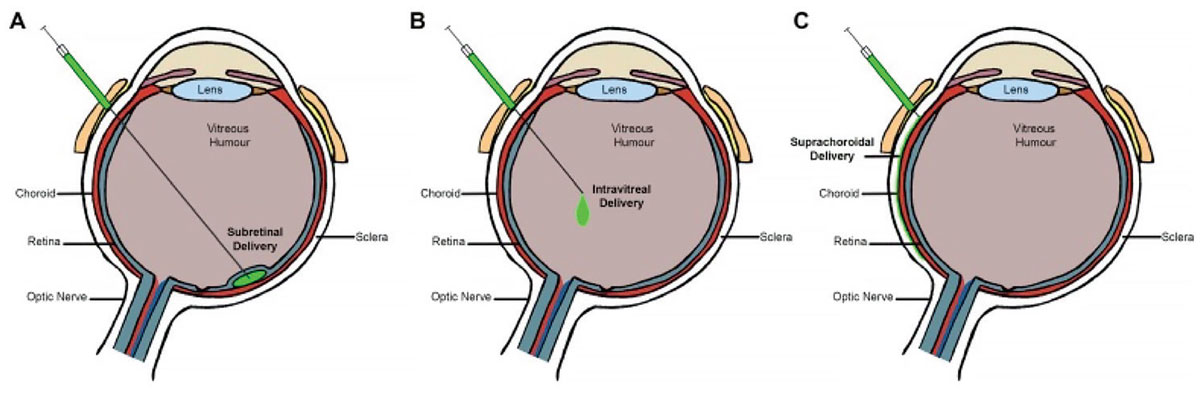
The standard for ocular gene therapy administration is subretinal injection; however, suprachoroidal and intravitreal gene delivery are being investigated as alternative routes of administration to avoid the potential complications of pars plana vitrectomy-associated procedures, such as macular hole and/or potential photoreceptor disruption associated with bleb creation, retinotomy with hemorrhage and/or fibrosis, retinal tear and detachment and cataract (Figure 1).6 Suprachoroidal delivery also offers a targeted, compartmentalized delivery of gene therapy to the chorioretina while minimizing exposure to the vitreous and anterior segment which may elicit an immune and inflammatory response.7-10
 |
|
Figure 1. Routes of ocular gene therapy administration: subretinal delivery (A); intravitreal delivery (B); suprachoroidal delivery (C). From Guimaraes TAC, Georgiou M, Bainbridge JWB, Michaelides M. Br J Ophthalmol. 2021;105(2):151-157.11 Licensed for reuse under the Creative Commons Attribution (CC BY) license. |
ABBV-RGX-314
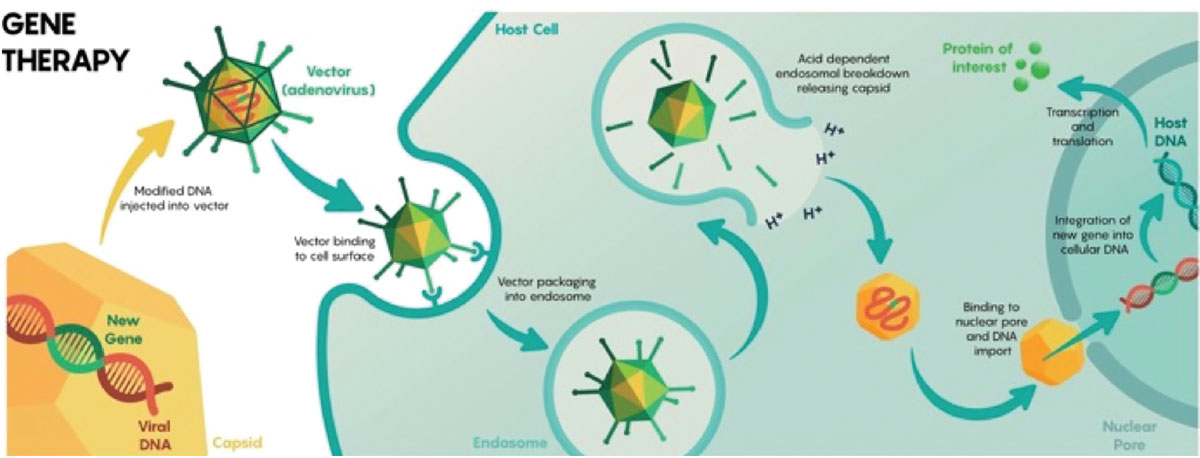
ABBV-RGX-314 is a novel gene therapy intervention being developed by RegenxBio and AbbVie that delivers a transgene encoding a ranibizumab-like anti-VEGF monoclonal antibody fragment to the retina through an adeno-associated virus (AAV) 8 vector (Figure 2). The administration of the therapy entails a single subretinal or suprachoroidal injection, strategically designed to produce enduring cellular expression of anti-VEGF therapy within the retinal tissues.
A Phase I/IIa, open-label, multiple-cohort, dose-escalation study (NCT03066258) reported positive safety and efficacy results with the subretinal delivery of ABBV-RGX-314 in nAMD. ABBV-RGX-314 was well-tolerated at all five doses and demonstrated stability of best-corrected visual acuity and central retinal thickness at six months.12
 |
|
Figure 2. Overview of adenovirus-mediated delivery of recombinant genetic material to host cells for endogenous expression of a desired protein. (From: https://commons.wikimedia.org/wiki/File:Viral_mediated_delivery_of_genes_to_neurons_1.jpg.16 Licensed for reuse under the Creative Commons Attribution-Share Alike 4.0 International license.) |
There are currently two ongoing Phase IIb/III trials: ATMOSPHERE (NCT04704921) and ASCENT (NCT05407636), investigating subretinal ABBV-RGX-314 in nAMD.13,14 The ATMOSPHERE (Phase IIb/III) and ASCENT (Phase III) trials are randomized, partially masked, controlled studies evaluating two dose levels of ABBV-RGX-314 against monthly intravitreal ranibizumab and bimonthly intravitreal aflibercept, respectively. The primary outcome of the trials is the mean change in BCVA from baseline to 54 weeks. RegenxBio has plans for the trials to support a global regulatory submission in late 2025 and first half of 2026.15
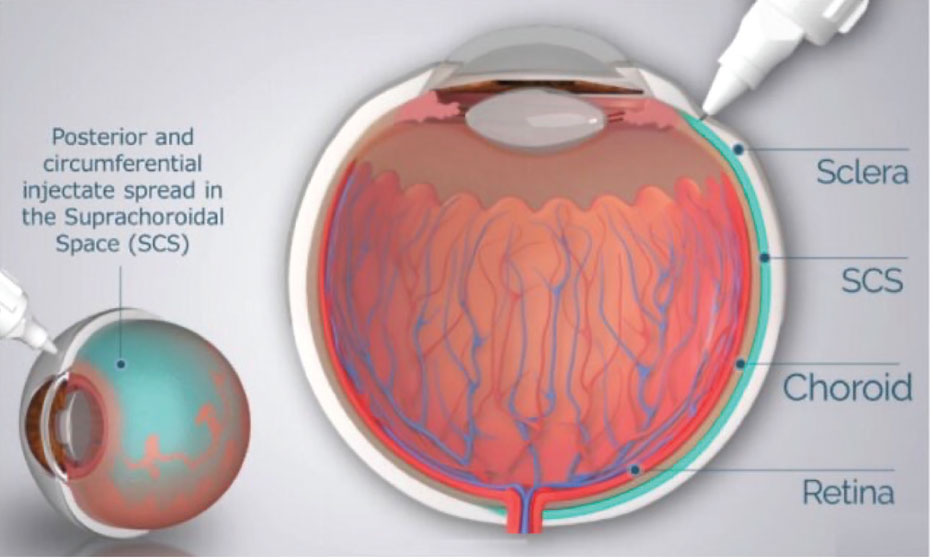
Simultaneously, RegenxBio is evaluating the suprachoroidal delivery of ABBV-RGX-314 in individuals with nAMD. AAVIATE (NCT04514653) is a multicenter, open-label, randomized, active-controlled, dose-escalation, Phase II trial investigating the efficacy, safety and tolerability of suprachoroidal delivery of ABBV-RGX-314 using the Clearside Suprachoroidal Space (SCS) Microinjector in comparison to monthly intravitreal ranibizumab (Figure 3).
Three dose levels of ABBV-RGX-314 are included in the trial at 2.5x1011 (Cohort 1), 5x1011 (Cohorts 2 and 3), and 1x1012 (Cohorts 4 to 6) genomic copies per eye. The primary efficacy endpoint is the mean change in BCVA at week 40 from baseline, with secondary endpoints including mean change in central retinal thickness and number of anti-VEGF injections following administration.
 |
|
Figure 3. Schematic of microneedle injection into the suprachoroidal space (SCS). From Wan C-R, Muya L, Kansara V, Ciulla TA. Pharmaceutics. 2021;13:288.7 (Licensed for reuse under the Creative Commons Attribution (CC BY) license.) |
Encouragingly, RegenxBio recently announced interim data that more than 50 patients in Cohorts 4 through 6 achieved an 80-percent reduction in annualized injection rate and a 50 percent injection-free rate through six months following a single suprachoroidal injection of ABBV-RGX-314.17
Furthermore, the therapy was well-tolerated across more than 100 patients from the three dose levels with no drug-related serious adverse events. Mild intraocular inflammation was reported at similar incidence rates in the first and second dose levels, while mild to moderate intraocular inflammation was reported at the third dose level in Cohorts 4 and 5; all of which resolved with topical corticosteroids. Of note, however, patients at the third dose level who received a short course of prophylactic topical steroids (Cohort 6) displayed no cases of intraocular inflammation.17 These early results support ABBV-RGX-314’s potential as a one-time, in-office treatment that may offer long-term durability and safety in nAMD.
Ixo-vec
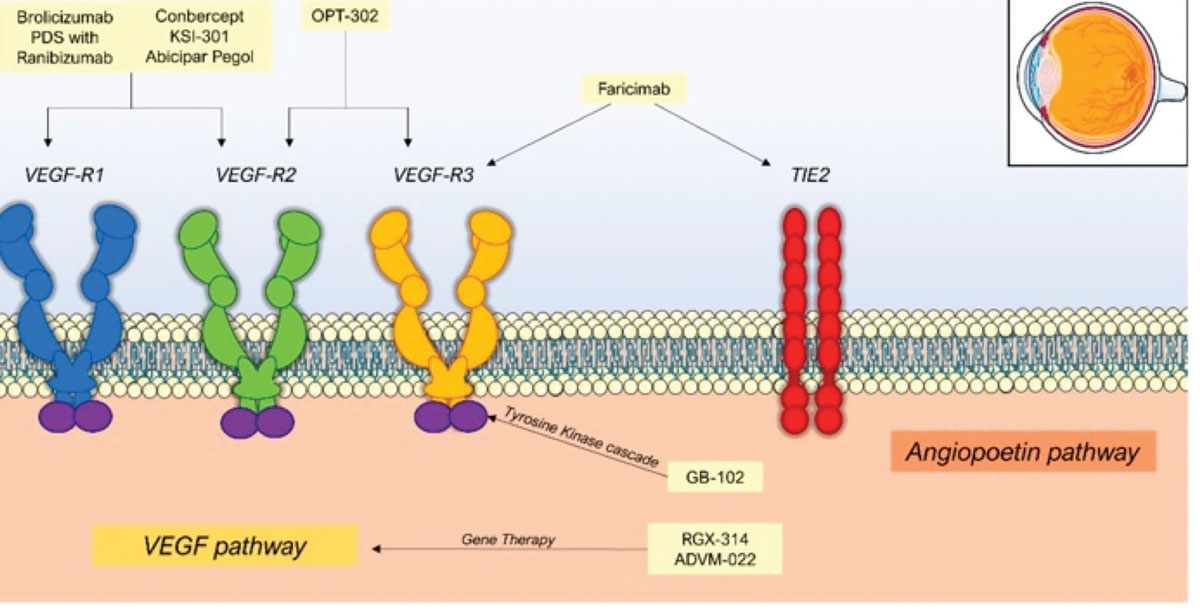
Ixoberogene soroparvovec (ixo-vec, formerly ADVM-022) (Adverum Biotechnologies) is a single-use intravitreal gene therapy that employs the AAV2.7m8 capsid for efficient retinal transduction and expression of the proven anti-VEGF protein, aflibercept (Figure 4).18,19
OPTIC (NCT03748784) was a two-year, open-label, prospective, multicenter Phase I study investigating the safety and tolerability of ixo-vec in non-naive nAMD.20 Patients were assigned to four cohorts differing by dose (2x1011 or 6x1011 vector genomes per eye [vg/eye]) and prophylactic steroids (oral prednisone vs. topical difluprednate).
The majority of ocular treatment-emergent adverse events (TEAEs) were dose-dependent and mild (84 percent) to moderate (16 percent) in severity, with anterior chamber cell and vitreous cell the most commonly reported.21 Five serious TEAEs were reported, with two deemed probably related to ixo-vec, including an asymmetric progression of dry AMD and a case of recurrent uveitis.
Notably, the high-dose group and low-dose group had 98 percent and 80 percent reduction in annualized anti-VEGF injection burden, respectively, and 80 percent and 53 percent injection-free rate, respectively.21 Furthermore, both dose groups displayed maintenance of BCVA and CRT measurements throughout the study. The superior safety and similar efficacy of the 2x1011 vg/eye dose relative to the 6x1011 vg/eye dose in the OPTIC trial supported continued evaluation of the latter dose in nAMD.
 |
|
Figure 4. Mechanisms of action of therapies for nAMD, including novel gene therapies in development. (From: Patel P, Sheth V. J. Clin. Med. 2021, 10, 2436.22 Licensed for reuse under the Creative Commons Attribution (CC BY) license.) |
LUNA (NCT05536973) is an ongoing Phase II trial investigating the safety and efficacy of ixo-vec at the 2x1011 vg/eye dose and a lower dose of 6x1010 vg/eye, in combination with enhanced corticosteroid prophylaxis.
Adverum recently announced positive preliminary results from the LUNA study, with both doses demonstrating maintenance of visual and anatomic outcomes. At 26 weeks, the 2x1011 and 6x1010 vg/eye doses achieved annualized reduction in anti-VEGF injection rates of 94 percent and 90 percent, respectively, and injection-free rates of 85 and 68 percent, respectively.23 Both doses displayed a maintenance of functional and anatomical outcomes from baseline to 26 weeks, in addition to apparent improved inflammatory profiles with corticosteroid prophylaxis compared to those from the OPTIC study.
These early results support the potential of a single in-office intravitreal injection of ixo-vec as a possible paradigm shift in nAMD treatment away from frequent anti-VEGF injections to durable and effective cellular-based biofactories.
4D-150
4D-150 (4D Molecular Therapeutics) is a single-use, low-dose intravitreal gene therapy using an evolved vector, R100, and a transgene cassette that expresses both aflibercept and a VEGF-C inhibitory RNAi to inhibit a total of four antiangiogenic factors: VEGF A; B; C; and placental growth factor (PlGF).24
PRISM (NCT05197270) is a prospective, multicenter, Phase I/II dose-escalation and randomized, controlled, masked expansion trial investigating the safety and tolerability of 4D-150 in nAMD.25 Phase I PRISM results revealed that all three dose cohorts of 3×1010, 1×1010, and 6×109 vg/eye were safe and well-tolerated in the dose exploration phase of the study.26 Furthermore, in the 3×1010 vg/eye cohort, patients experienced a 96.7-percent overall reduction in the mean annualized anti-VEGF injection rate and four of five eyes were supplemental aflibercept-free.
Interim Phase II PRISM results met all endpoints through 24 weeks in nAMD patients with severe disease activity and high treatment burden.27 4D-150 at a dose of 3×1010 vg/eye showed equivalent and stable BCVA outcomes and improved retinal anatomical control with reduced central subfield thickness variability compared to the bimonthly aflibercept arm, in addition to an 89 percent overall reduction in annualized anti-VEGF injection rate and a 63 percent injection-free rate.
Notably, a favorable safety profile was achieved with no significant or recurrent intraocular inflammation with a 20-week prophylactic topical corticosteroid taper. Following the positive initial results from PRISM, the FDA and European Medicines Agency granted the RMAT and Priority Medicines (PRIME) designations, respectively, to 4D-150 with the goal of increasing collaboration on regulatory approval planning and expediting drug development.24
Bottom line
Gene therapy holds the potential to alleviate the treatment burden in nAMD by delivering sustained and enduring therapeutic effects through the production of endogenous anti-VEGF within the retina. Many challenges face the ongoing development of these therapies, yet significant progress has been realized regarding their routes of administration, safety profiles and efficacy outcomes.
The prospect of reduced treatment frequency offers benefits for patients, including substantial enhancements in quality of life, vision preservation and a reduction in the socio-economic impact associated with vision impairment. Viral-vector gene therapy appears to be a particularly promising avenue and the aforementioned therapies support the great potential in advancing the treatment landscape for nAMD. RS
REFERENCES
1. Campbell RJ, Bronskill SE, Bell CM, et al. Rapid expansion of intravitreal drug injection procedures, 2000 to 2008: A population-based analysis. Arch Ophthalmol Chic Ill. 2010;128:359–362.
2. Peden MC, Suñer IJ, Hammer ME, et al. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122:803.
3. Moshfeghi AA, Pitcher JD, Lucas G, et al. Visual acuity outcomes in patients receiving frequent treatment of neovascular age-related macular degeneration in clinical practice. J Vitreoretin Dis. 2021;5:221–226.
4. Ciulla TA, Hussain RM, Pollack JS, et al. Visual acuity outcomes and anti–vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: A real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4:19–30.
5. Khanani AM, Skelly A, Bezlyak V, et al. SIERRA-AMD: A retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2020;4:122–133.
6. Peng Y, Tang L, Zhou Y. Subretinal injection: A review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58:217–226.
7. Wan C-R, Muya L, Kansara V, et al. Suprachoroidal delivery of small molecules, nanoparticles, gene and cell therapies for ocular diseases. Pharmaceutics. 2021;13:288.
8. Hancock SE, Wan C-R, Fisher NE, et al. Biomechanics of suprachoroidal drug delivery: From benchtop to clinical investigation in ocular therapies. Expert Opin Drug Deliv. 2021;18:777–788.
9. Kansara V, Muya L, Wan C, et al. Suprachoroidal delivery of viral and nonviral gene therapy for retinal diseases. J Ocul Pharmacol Ther. 2020;36:384–392.
10. Kansara VS, Cooper M, Sesenoglu-Laird O, et al. Suprachoroidally delivered DNA nanoparticles transfect retina and retinal pigment epithelium/choroid in rabbits. Transl Vis Sci Technol. 2020;9:21.
11. Guimaraes TAC de, Georgiou M, Bainbridge JWB, et al. Gene therapy for neovascular age-related macular degeneration: Rationale, clinical trials and future directions. Br J Ophthalmol. 2021;105:151–157.
12. ClinicalTrials.gov. Safety and tolerability of RGX-314 (investigational product) gene therapy for neovascular AMD trial. https://www.clinicaltrials.gov/study/NCT03066258?tab=results. Accessed January 18, 2024.
13. ClinicalTrials.gov. Pivotal 1 Study of RGX-314 gene therapy in participants with nAMD. https://clinicaltrials.gov/study/NCT04704921. Accessed January 18, 2024.
14. ClinicalTrials.gov. Pivotal 2 study of RGX-314 gene therapy in participants with nAMD. https://clinicaltrials.gov/study/NCT05407636. Accessed January 18, 2024.
15. RegenxBio. RegenxBio announces updated strategic plans and third quarter 2023 financial results. Available from: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-updated-strategic-plans-and-third-quarter/. Accessed January 18, 2024.
16. Wikimedia Commons, 2022. Viral mediated delivery of genes to neurons 1.jpg. https://commons.wikimedia.org/wiki/File:Viral_mediated_delivery_of_genes_to_neurons_1.jpg. Accessed February 17, 2024.
17. RegenxBio. RegenxBio announces positive interim data from phase II AAVIATE trial of ABBV-RGX-314 for the treatment of wet AMD using suprachoroidal delivery. https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-positive-interim-data-phase-ii-aaviater/. Accessed January 17, 2024.
18. Grishanin R, Vuillemenot B, Sharma P, et al. Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol Ther J Am Soc Gene Ther. 2019;27:118–129.
19. Gelfman CM, Grishanin R, Bender KO, et al. Comprehensive preclinical assessment of ADVM-022, an intravitreal anti-VEGF gene therapy for the treatment of neovascular AMD and diabetic macular edema. J Assoc Ocul Pharmacol Ther. 2021;37:181–190.
20. ClinicalTrials.gov. ADVM-022 intravitreal gene therapy for wet AMD. https://clinicaltrials.gov/study/NCT03748784. Accessed January 18, 2024.
21. Khanani AM, Boyer DS, Wykoff CC, et al. Safety and efficacy of ixoberogene soroparvovec in neovascular AMD in the United States (OPTIC): A prospective, two-year, multicentre phase 1 study. EClinicalMedicine. 2024;67:102394.
22. Patel P, Sheth V. New and innovative treatments for neovascular AMD. J Clin Med. 2021;10:2436.
23. Adverum Biotechnologies. Adverum Biotechnologies announces positive preliminary efficacy and safety data from LUNA Phase 2 trial of ixo-vec in patients with wet AMD. https://investors.adverum.com/news/news-details/2024/Adverum-Biotechnologies-Announces-Positive-Preliminary-Efficacy-and-Safety-Data-from-LUNA-Phase-2-Trial-of-Ixo-vec-in-Patients-with-Wet-AMD/default.aspx. Accessed February 17, 2024.
24. 4DMT. 4DMT receives FDA regenerative medicine advanced therapy (RMAT) designation for 4D-150 genetic medicine for intravitreal treatment of wet AMD, the First RMAT designation in wet AMD. GlobeNewswire News Room. 2023. https://www.globenewswire.com/news-release/2023/12/21/2799961/0/en/4DMT-Receives-FDA-Regenerative-Medicine-Advanced-Therapy-RMAT-Designation-for-4D-150-Genetic-Medicine-for-Intravitreal-Treatment-of-Wet-AMD-the-First-RMAT-Designation-in-Wet-AMD.html. Accessed January 18, 2024.
25. ClinicalTrials.gov. 4D-150 in patients with neovascular (wet) age-related macular degeneration. https://clinicaltrials.gov/study/NCT05197270. Accessed January 18, 2024.
26. Khanani AM, Hershberger VS, Kay CN, et al. Interim results for the Phase 1/2 PRISM Trial evaluating 4D-150, a dual-transgene intravitreal genetic medicine in individuals with neovascular (wet) age-related macular degeneration. Invest Ophthalmol Vis Sci. 2023;64:5055.
27. 4DMT presents positive interim data from randomized phase 2 PRISM clinical trial of intravitreal 4D-150 demonstrating favorable tolerability & clinical activity in wet AMD. https://ir.4dmoleculartherapeutics.com/news-releases/news-release-details/4dmt-presents-positive-interim-data-randomized-phase-2-prism/. Accessed February 4, 2024.



