Take-home points
|
 |
Bios Dr. Clark is a vitreoretinal surgical fellow at Retina Vitreous Associates/University of Southern California Roski Eye Institute, Los Angeles. Dr. Steinle is a retina specialist with California Retina Consultants in the Central Coast region of California. DISCLOSURES: Dr. Clark has no relevant disclosures. Dr. Steinle disclosed relationships with Appelis Pharmaceuticals, Genentech/Roche, Novartis and Regeneron Pharmaceuticals. |
The treatment paradigm for geographic atrophy associated with advanced age-related macular degeneration has changed rapidly in the past year thanks to the approval of two new intravitreal injections, pegcetacoplan (Syfovre, Apellis Pharmaceuticals) and avacincaptad pegol (Izveray, Iveric Bio/Astellas). These new medications inhibit the complement cascade implicated in development of retinal pigment epithelium cell death leading to GA.1–3
Several other medications are also being developed in the therapeutic pipeline, and we may someday have gene therapy products, suprachoroidal drugs and other novel biologics as part of our armamentarium against this devastating disease.4,5 As these new treatment options enter into our clinical practice, we’ll face new challenges for safe and effective care delivery. Here, we describe our technique for performing safe intravitreal injection of pegcetacoplan and avacincaptad pegol.
Indications for injection
Pegcetacoplan should be given every 25 to 60 days. In clinical trials, pegcetacoplan was shown to reduce the rate of GA lesion growth 16 to 22 percent in the first 12 months of treatment, depending on whether patients receive monthly or bimonthly injections,6 with an enhanced reduction effect of 25 to 35 percent in lesion growth rate in years two and three.7,8
Avacincaptad pegol has the same indication. It’s approved for monthly dosing up to one year and, in clinical trials, demonstrated an 18-to-35-percent reduction in the GA lesion growth rate over the first 12 months of treatment.9,10
Before the injection
As with any intervention, careful discussion regarding the risks and benefits with patients is paramount before initiating therapy. Both pegcetacoplan and avacincaptad pegol have known risks that patients should be told about before the injection. Besides risks inherent to any intravitreal injection, namely endophthalmitis and damage to other structures of the eye, including retinal detachment, both medications have several notable additional risks. They include conversion to wet macular degeneration, nonartertic ischemic optic neuropathy, and a greater likelihood of transient elevated intraocular pressure.6–10
 |
|
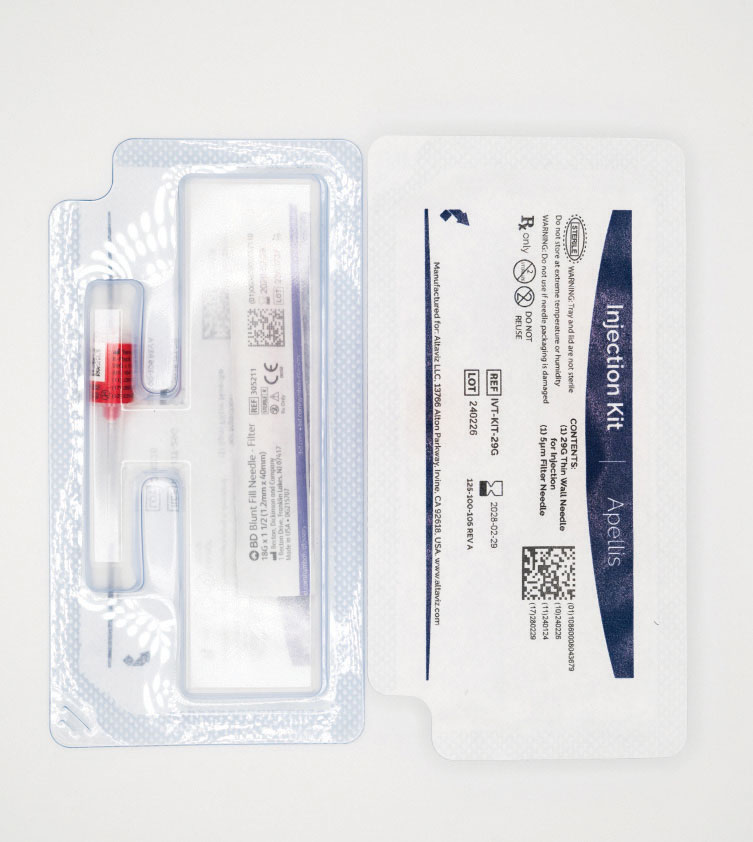
Figure 1. Pegcetacoplan (Syfovre) comes with an accompanying injection kit that contains an 18G filter needle and a 29G thin-walled Luer-lock needle. (Courtesy: Apellis Pharmaceuticals) |
Complement inhibition has also been associated with rare instances of retinal vasculitis following the first administration of the medication.11,12 Data are still being gathered to better define this risk profile, but unofficial reported estimates place the incidence of retinal vasculitis at around 0.01 percent for pegcetacoplan. Apellis has recommended switching from a previously supplied 19G filter needle to an 18G filter needle when preparing the injection.
Preparing the injection
On the day of the injection, the eye should be inspected to ensure it’s free of both infection and active inflammation. Both medications should be stored at temperatures between 2 and 8C until they’re used and allowed to reach room temperature prior to the injection, which eliminates the increased viscosity associated with colder storage temperatures. Vials should not be frozen or shaken, and vials should only be used for a single injection for a single eye in a single patient. The medications should appear as a colorless to light yellow liquid. If the vial contains any particulate, turbidity or discoloration, it should be discarded.
Pegcetacoplan is a 0.1 mL injection of a 150 mg/mL solution, providing 15 mg of drug into the vitreous cavity. The medication is distributed in a vial, with an accompanying injection kit that contains an 18G filter needle and a 29G thin-walled Luer-lock needle (Figure 1). The injection also requires a sterile 1-cc Luer-lock syringe and alcohol swab. A sterile 27G needle with Luer-lock can also be used in lieu of the thin-walled 29G needle.13
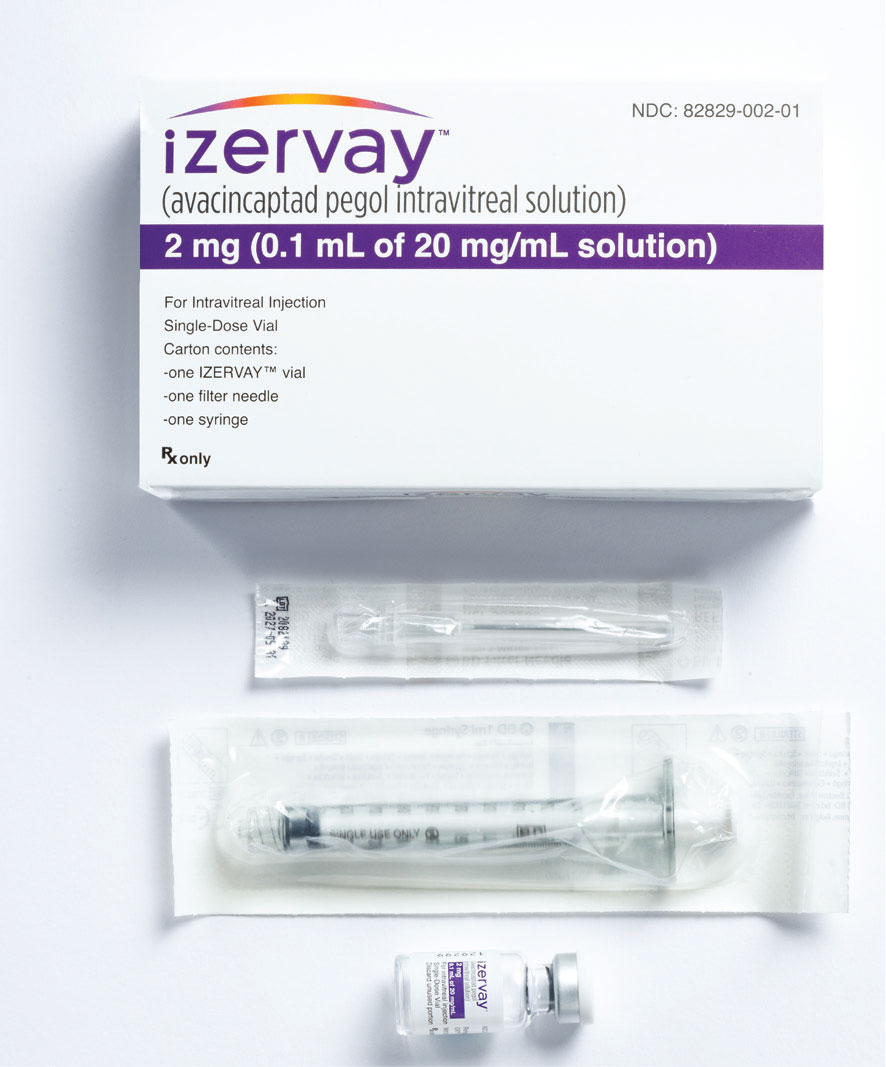
Avacincaptad pegol is 0.1-mL injection of a 20-mg/mL solution for a total of 2 mg of drug per injection. The medication vial is packaged with a 19G filter needle and sterile 1-cc Luer-lock syringe. The injection can be performed with a 30G needle (Figure 2, page 20).14
Similarities between the two injections
The following procedures we describe apply to both treatments. They include:
1) Remove the cover from the vial cap and clean the septum with an alcohol swab.
2) Transfer the medication from the vial to the syringe using the supplied filter needle. Sitting the vial upright for one minute prior to withdrawing the medication can help the liquid accumulate at the base of the vial and make the transfer easier. Tilting the vial slightly to pool the liquid in one corner will also limit introducing air bubbles during withdrawal.
3) Exchange the filter needle for the injection needle (29G thin-walled needle for pegcetacoplan, 30G for avacincaptad pegol) and discard excess medication until 0.1 mL remains in the syringe. This step is critical to expel any air bubbles within the solution.
For pegcetacoplan, don’t shake or tap the syringe. This will cause more bubbles to form within the solution. Rather, perform a smooth and controlled withdrawal and advancement (charging) of the plunger until no bubbles are visible within the syringe.
For avacincaptad pegol, lightly tapping the side of the syringe will bring the bubbles to the top, where they can be expelled.
Cleaning the ocular surface
As with all intravitreal injections, the ocular surface should be cleaned meticulously with an antiseptic agent. One suggestion is to press a sterile, betadine-soaked cotton tip against the globe at the intended injection site until an indentation is visible on the globe surface. By holding this position for 10 to 30 seconds, we hope to soften the globe to better accommodate the 0.1-mL injection volume. With this decompression technique, we have seen few significant IOP elevations immediately after the injection.
Injection technique for pegcetacoplan
One of the unique aspects of pegcetacoplan is its increased viscosity relative to other common intravitreal injections. The medication’s thick consistency will cause it to move more slowly through the filter needle and may require increased back pressure on the syringe to remove the fluid from the vial. This increased viscosity also plays a role when performing the injection. Apply continued pressure to the syringe plunger when performing the injection to express the medication. The thin-walled, larger 29G needle is provided to aid with fluid flow and the Luer locks provide an extra layer of security during the injection.
 |
|
Figure 2. Avacincaptad pegol (Izervay) comes with an injection kit that includes a glass vial, a sterile 5-µm filter needle (19G x 1.5 inch) and an empty sterile 1-mL Luer lock syringe with a 0.1-mL dose mark. The glass vial, filter needle and empty syringe are for single use only. (Courtesy Iveric Bio/Astellas) |
Stabilizing hand-holding of the syringe also aids in achieving a safe injection. We typically use a bimanual technique. Hold the syringe in the dominant hand while, with the nondominant hand, lightly holding around the distal end of the syringe to stabilize the injection. Once the needle is within the vitreous, use the thumb of the nondominant hand to slowly and firmly depress the plunger. We recommend injecting over about five seconds.
While withdrawing the needle, a sterile cotton-tip applicator can tamponade the wound to minimize medication reflux. Because the injection push takes longer than other intravitreal injections, a lid speculum can minimize the chance of eyelash contamination of the injection needle.
Once the injection is completed, inspect the optic nerve to confirm it’s perfused and that vision is at least counting fingers. If the nerve is pale and vision declines to no light perception, consider an anterior chamber paracentesis to reduce the elevated IOP. If the vision declines but the nerve remains perfused with venous pulsations, the patient should remain in the clinic and rechecked in five to 10 minutes as the vision and perfusion will often improve on their own. Failure for the vision to improve after brief observation should prompt an anterior chamber paracentesis.
Injection technique for avacincaptad pegol
Avacincaptad pegol has a viscosity similar to that of anti-VEGF agents. Thus, it doesn’t have the same considerations in regard to medication preparation and injection pressure as pegcetacoplan, but it still comes with an accompanying injection kit (Figure 2). A 30G needle is recommended for the injection. Smaller-bore needles may limit fluid flow. Avacincaptad pegol is also a 0.1-mL injection, so it’s important to monitor the patient for an immediate postinjection IOP spike and to intervene.
Bottom line
With minor adjustments to the typical intravitreal injection techniques, vitreoretinal specialists can safely and reproducibly deliver pegcetacoplan and avacincaptad pegol. RS
REFERENCES
1. Shughoury A, Sevgi DD, Ciulla TA. The complement system: A novel therapeutic target for age-related macular degeneration. Expert Opin. Pharmacother. 2023;24:1887-1899.
2. Liao DS, Grossi FV, Mehdi DE, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: A randomized Phase 2 trial. Ophthalmology. 2019:127:186–195.
3. Jaffe GJ, Westby K, Csaky KG. et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: A randomized pivotal Phase 2/3 trial. Ophthalmology. 2020;128:576–586.
4. Mahmoudzadeh R, Hinkle JW, Hsu J, Garg SJ. Emerging treatments for geographic atrophy in age-related macular degeneration. Curr Opin Ophthalmol. 2021;32:294–300.
5. Khan H, Aziz AA, Sulahria H, et al Emerging treatment options for geographic atrophy (GA) secondary to age-related macular degeneration. Clin. Ophthalmol. 2023;17:321–327.
6. Goldberg R, Heier JS, Wykoff CC, et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the Phase 3 OAKS and DERBY studies. Inv Ophthalmol Vis Sci. 2022;63:1500.
7. Heier JS, Lad EM, Holz FG, et al Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): Two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402:1434–1448.
8. Steinle NC. Efficacy of pegcetacoplan in patients with geographic atrophy. Paper presented at the American Society of Retina Specialists 41st Annual Scientific Meeting. July 30, 2023; Seattle, WA.
9. Patel SS, Lally DR, Hsu J, et al. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye (Lond). 2023;17:3551-3557.
10. Khanani AM, Patel SS, Staurenghi FG, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet. 2023;402:1449–1458.
11. American Society of Retina Specialists e-mail to members reporting cases of vasculitis following pegcetacoplan injection. July 15, 2023.
12. Cruz-Pimentel M, Wu L. Complement inhibitors for advanced dry age-related macular degeneration (geographic atrophy): Some light at the end of the tunnel? J. Clin. Med. 2023;12:5131.
13. Syfovre [package insert]. Waltham, MA: Apellis Pharmaceuticals; 2023.
14. Izervay [package insert]. Tokyo: Iveric Bio; 2023.



