Take-home points
|
 |
Bios Drs. Alejo and Pinzon practice at the Eye and Vision Institute in the Medical City, Philippines. Dr. Silva is an associate professor of ophthalmology at Harvard Medical School and assistant chief of telemedicine at the Joslin Diabetes Center in Boston. DISCLOSURES: Drs. Alego and Pinzon: No disclosures Dr. Silva: Research support: Optos; Optomed; Kubota Vision. |
Though diabetic retinopathy screening is a crucial aspect of maintaining patients’ vision, it’s fraught with challenges. The current obstacles to more effective DR screening include limited access to specialized health care and a shortage of ophthalmologists or trained screeners. The high cost of screening technology and lack of awareness about the importance of early detection also contribute to low screening rates. Additionally, the variability in disease progression and the need for regular, often lifelong, monitoring complicate consistent follow-up.
Implementing and maintaining effective screening programs also faces logistical and financial barriers. These challenges collectively hinder early diagnosis and timely treatment, critical for preventing vision loss in diabetic patients. Here, we’ll describe how artificial intelligence may help ease some of this screening burden.
Principles of AI in DR and DME Screening/Monitoring
Recent innovations in artificial intelligence are revolutionizing screening and monitoring of DR and DME. The principles behind AI in this context involve several components that enable effective and efficient detection of DR and DME:
• Data collection and preprocessing. AI systems rely on large datasets of retinal images for training. These images are often obtained from diverse populations to ensure the model can generalize well across different demographic groups. Preprocessing steps include normalization, resizing and enhancement of images to improve the quality and consistency of the input data.1
• Machine learning algorithms. At the core of AI for DR screening are machine learning algorithms, particularly convolutional neural networks. CNNs are designed to automatically and adaptively learn spatial hierarchies of features from input images.2 In the context of DR, these features may include minute details such as microaneurysms, hemorrhages, exudates and neovascularization. For DME, these include macular thickness and structural changes from optical coherence tomography imaging.
• Training the model. The training process involves feeding the neural network a curated dataset of labeled retinal images, where the presence and severity of DR have been annotated by expert ophthalmologists.3 The model learns to recognize patterns and correlations between the retinal features and the labels through iterative optimization techniques (Figure 1). During training, the model’s parameters are adjusted to minimize the error in its predictions.
• Validation and testing. After training, the model’s performance is validated using a separate set of images not seen during training. This step helps in tuning the model parameters and avoiding overfitting. The final evaluation involves testing the model on a completely independent dataset to assess its accuracy, sensitivity, specificity and overall robustness in detecting DR.4
• Deployment and integration. Once validated, the AI model can be deployed in clinical settings. It can be integrated into existing health-care workflows and screening programs. Typically, retinal images are captured using fundus cameras and then analyzed by the AI system, which can provide rapid, automated diagnoses.5 The system highlights images with signs of DR, prioritizing cases that need urgent attention from ophthalmologists. However, it’s important to emphasize that regulatory requirements for AI applications remain and a clinical trial at the level required by the FDA will be needed for fully autonomous use in the United States.
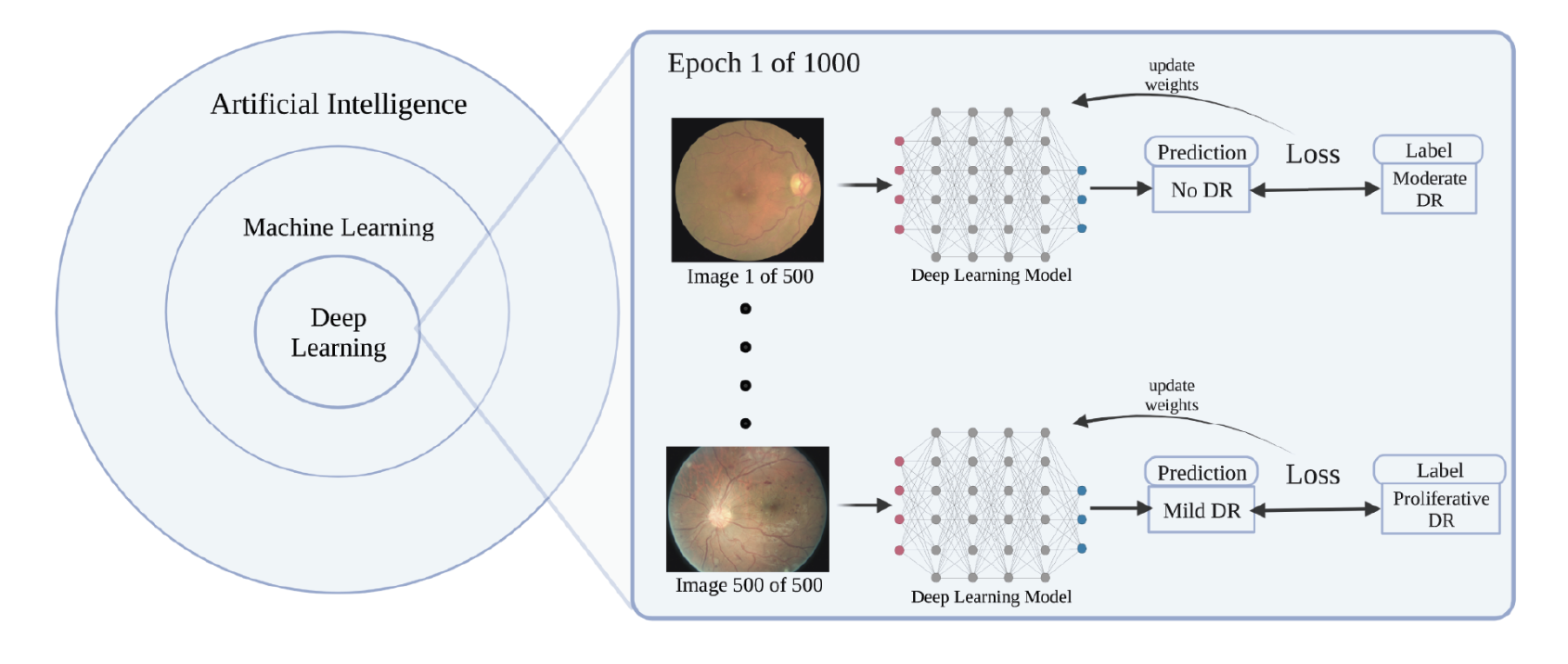 |
| Figure 1. An example of a deep learning model being trained to classify diabetic retinopathy severity using a dataset of 500 images. (Image taken from: Rajesh, AE, Davidson, OQ, Lee, C S, Lee AY. Artificial intelligence and diabetic retinopathy: AI framework, prospective studies, head-to-head validation, and cost-effectiveness. Diabetes Care 2023;46:10:1728–1739.) |
Clinical Trials of AI
Several clinical trials have demonstrated the efficacy and reliability of artificial intelligence in screening and monitoring DR and DME. Processing of data for DR and DME requires different approaches. DR is identified by photographic imaging while DME relies on macular thickness measurements and structural changes from OCT imaging. The following trials highlight AI’s role for enhancing diagnostic accuracy, predicting disease progression and optimizing treatment strategies for screening and monitoring DR and DME:
• Google Health’s AI system for diabetic retinopathy and DME. A significant study by Google Health and published in JAMA Ophthalmology, developed and validated an AI system to detect DR and DME from retinal images and OCT scans. The AI system used in this study demonstrated high sensitivity and specificity in detecting DME. It concluded that the model’s performance was comparable to that of expert ophthalmologists.3 This study highlighted the potential for AI to assist in screening programs, particularly in regions with limited access to specialized care.
• IDx-DR system for autonomous detection of diabetic retinopathy. The IDx-DR system, an AI-based diagnostic tool, underwent a pivotal clinical trial to evaluate its accuracy in detecting diabetic retinopathy, including macular edema, from retinal images. This clinical trial involved 900 patients across 10 primary care sites in the United States. The IDx-DR achieved sensitivity and specificity rates of 87.2 percent and 90.7 percent, respectively, for detecting more than mild diabetic retinopathy.3 This led to the IDx-DR system becoming the first FDA-approved AI device for autonomous diabetic retinopathy screening.
• Retinal Deep Learning system for OCT analysis. A study published in The Lancet Digital Health evaluated the performance of the Retinal Deep Learning (RDL) system, an AI-powered tool designed to analyze OCT scans for detecting DME and other retinal diseases. The RDL system was trained on a large dataset of OCT images and validated on an independent test set. This AI model demonstrated high accuracy in detecting DME, with sensitivity and specificity exceeding 90 percent.6 The results showed the potential for AI to improve early detection and monitoring of DME, aiding in timely treatment interventions.
• VoxelCloud AI platform for treatment response evaluation. VoxelCloud developed an AI platform to monitor treatment response in DME patients using OCT imaging. A clinical trial was conducted to assess the platform’s ability to track changes in macular thickness and fluid accumulation. The trial involved multiple clinical centers and included a diverse patient population. The results demonstrated accurate quantified changes in retinal morphology, which correlated well with clinical outcomes. The study highlighted the platform’s utility in evaluating treatment efficacy and adjusting management plans based on AI-driven insights.7
• Optovue’s AngioAnalytics for predictive analytics. Optovue’s AngioAnalytics, an AI-powered OCT system, was evaluated in a clinical trial to determine its effectiveness in predicting disease progression in DME patients. This trial demonstrated that the AI system could identify biomarkers associated with DME progression.8 Predictive analytics provided by AngioAnalytics may enable personalized monitoring schedules, improving patient management. The study underscored the role of AI in forecasting disease trajectory and optimizing follow-up protocols.
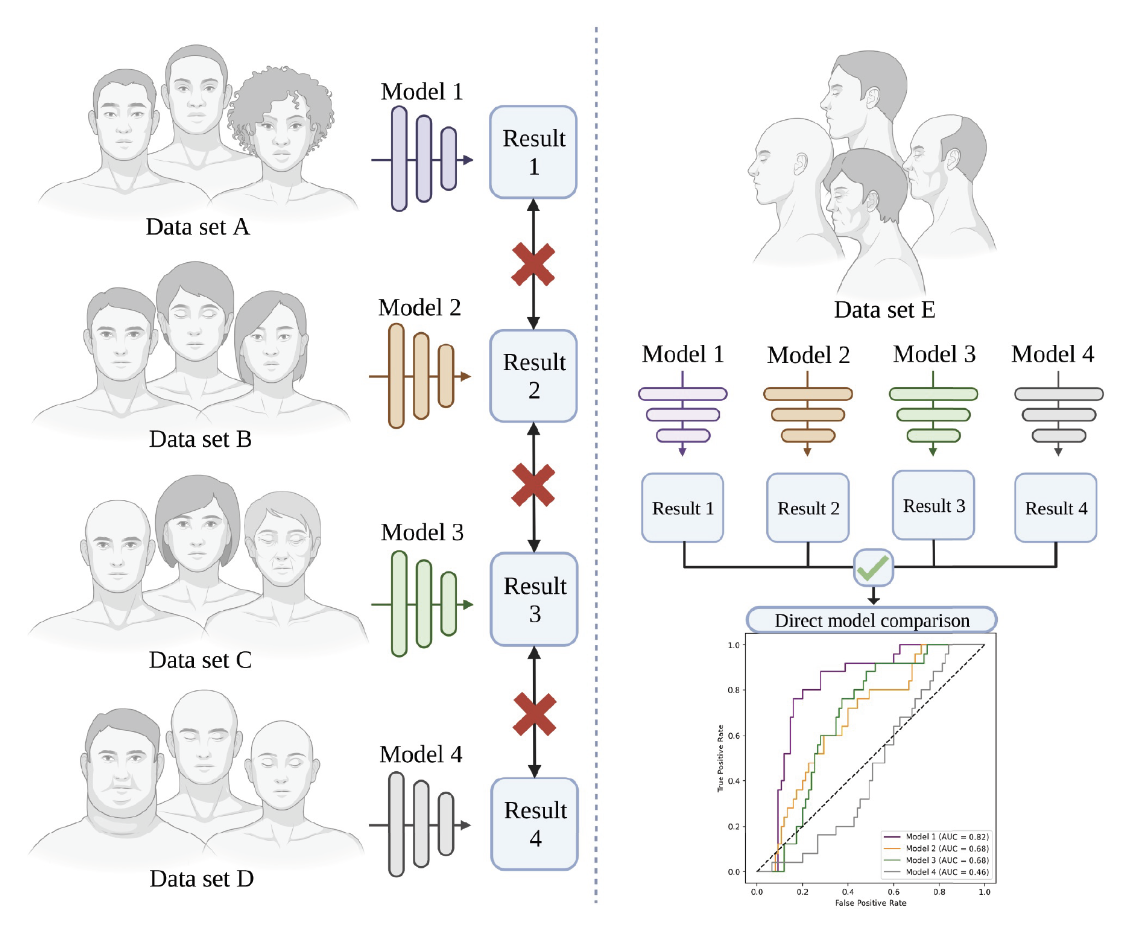 |
| Figure 2. Evaluation of different learning models using different datasets (left) make them incomparable. On the other hand, assessment of the same models using one dataset (right) allow their performance to be directly compared. (Image taken from: Rajesh AE, Davidson OQ, Lee CS, Lee AY. Artificial intelligence and Diabetic retinopathy: AI framework, prospective studies, head-to-head validation, and cost-effectiveness. Diabetes Care 2023;46:10:1728–1739.) |
Benefits and Challenges
AI-driven DR screening offers numerous benefits, including increased accessibility, especially in underserved areas with limited health-care resources. It provides a cost-effective and scalable solution for regular and widespread screening. Additionally, AI can reduce the burden on health-care professionals by automating routine analysis, allowing them to focus on complex cases.9
However, several challenges remain. Ensuring the AI models are trained on diverse datasets is crucial to avoid biases and ensure accuracy across different populations. Regulatory approvals and acceptance from medical professionals are essential for widespread adoption. Privacy and ethical considerations regarding the use of patient data must be addressed. Another crucial step is the head-to-head evaluation and comparison of the various models produced. Given that different models use information from different demographic groups, the performance of these may be affected depending on the given data sets (Figure 2).
In conclusion, these clinical trials and studies illustrate the transformative impact of AI-powered OCT in monitoring diabetic macular edema. AI systems enhance diagnostic accuracy, facilitate early detection, provide predictive insights and improve treatment response evaluation. As a result, they hold significant promise for improving clinical outcomes and patient care in diabetic retinopathy management. RS
1. Gulshan V, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016;316:22:2402-2410.
2. Ting DSW, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017;318:22:2211-2223.
3. Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digital Med 2018;1, 39.
4. Gargeya R, Leng T. Automated identification of diabetic retinopathy using deep learning. Ophthalmology 2017;124:7:962-969.
5. Valentina Bellemo, MSc, Zhan W Lim, PhD, Gilbert Lim, PhD, et al. Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: A clinical validation study. Lancet Digital Health. May 2019;1:1. Online publication.
6. De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nature Medicine 2018;24:9:1342-1350.
7. Hwang DK, Chou YB, Lin TC, Yang HY, Kao ZK, Kao CL, Yang YP, Chen SJ, Hsu CC, Jheng YC. Optical coherence tomography-based diabetic macula edema screening with artificial intelligence. J Chin Med Assoc 2020;83:11:1034-1038.
8. Lee CS, Tyring AJ, Wu Y, et al. Deep-learning based, automated segmentation of macular edema in optical coherence tomography. Biomedical Optics Express 2017;8:7:3440-3448.
9. Beede E, Baylor E, Hirsch F, et al. (2020). A human-centered evaluation of a deep learning system deployed in clinics for the detection of diabetic retinopathy. CHI ‘20: Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems. April 2020. Pages 1-12.10. https://doi.org/10.1145/3313831.3376718.



