Take-home points
|
 |
Bios Dr. Kunala is a research engineer in the department of ophthalmology at Stanford Medicine in Palo Alto, California. His current interests are in development of design and instrumentation of preclinical vision devices, to conduct noninvasive in vivo retinal imaging. Dr. Hunter is an associate professor in the School of Optometry and Vision Science at the University of Waterloo in Ontario, Canada. She has more than 17 years of experience in retinal imaging, including work with adaptive optics instrumentation and fluorescence lifetime imaging. DISCLOSURES: Dr. Kunala has no relevant financial relationships to disclose. Dr. Hunter has a patent pending through the University of Rochester related to a method for the collection of registered fluorescence lifetime data. |
The retinal pigment epithelium is a critical layer of the retina that supports healthy vision and is often thought of as the caretaker of the outer retina. Its dysfunction or malfunction is linked to several eye diseases, including age-related macular degeneration, Stargardt disease and retinitis pigmentosa. The ability to monitor changes in the molecular composition and function of the RPE will be highly significant for clinicians seeking early diagnosis and monitoring of treatment efficacy. Here, we’ll discuss an emerging imaging modality, fluorescence lifetime imaging ophthalmoscopy, or FLIO, which has the potential to help us detect diseases earlier than is currently possible.
Fundus autofluorescence and FLIO
Fundus autofluorescence is a widely used clinical tool1 that images naturally occurring fluorescent molecules (high-energy light is absorbed and then emitted with lower energy at a longer wavelength) within the RPE to visualize changes in the fluorescence intensity. Bisretinoid lipofuscin, a byproduct of molecules involved in the eye’s visual cycle, accumulates in the RPE with age, while melanin granules decrease over time. Lipofuscin builds up even faster in Stargardt disease and clumps together in AMD. FAF uses these molecules (lipofuscin and melanin) to observe intensity variations across the retina with age and disease.2
Beyond imaging fluorescence intensity, measurement of the time delay between fluorescence excitation and emission detection can provide insight into relative fluorescence contributions and the cellular environment. FLIO is a promising tool that compliments FAF.3 It’s a functional read-out that’s independent of the intensity of the fluorophore. Each fluorescent molecule (and states of molecules) has a unique decay time (“lifetime”) that depends on many factors and can vary with the wavelength of light used for excitation. When there are multiple molecules present, the overall measured lifetime reflects a relative combination of their individual lifetimes.
FLIO, with blue light excitation, used in clinical research has already shown differences in fluorescence lifetime between healthy eyes and those with various diseases.4 Studies have also found changes in lifetime with retinal eccentricity and age.5 As mentioned earlier, the main potential advantage for FLIO is the early detection of disease which might not be possible with conventional FAF. For example, researchers at the Moran Eye Center have detected FLIO variations in people with macular telangiectasia at early stages of disease before retinal damage has occurred.6 FLIO has also shown a characteristic ring-shaped pattern in people with AMD even in early stages.7 The possibility of earlier identification of patients at risk may be critical in the implementation of proposed preventative measures for disease such as vitamin and mineral supplementation or red light therapy.8
 |
|
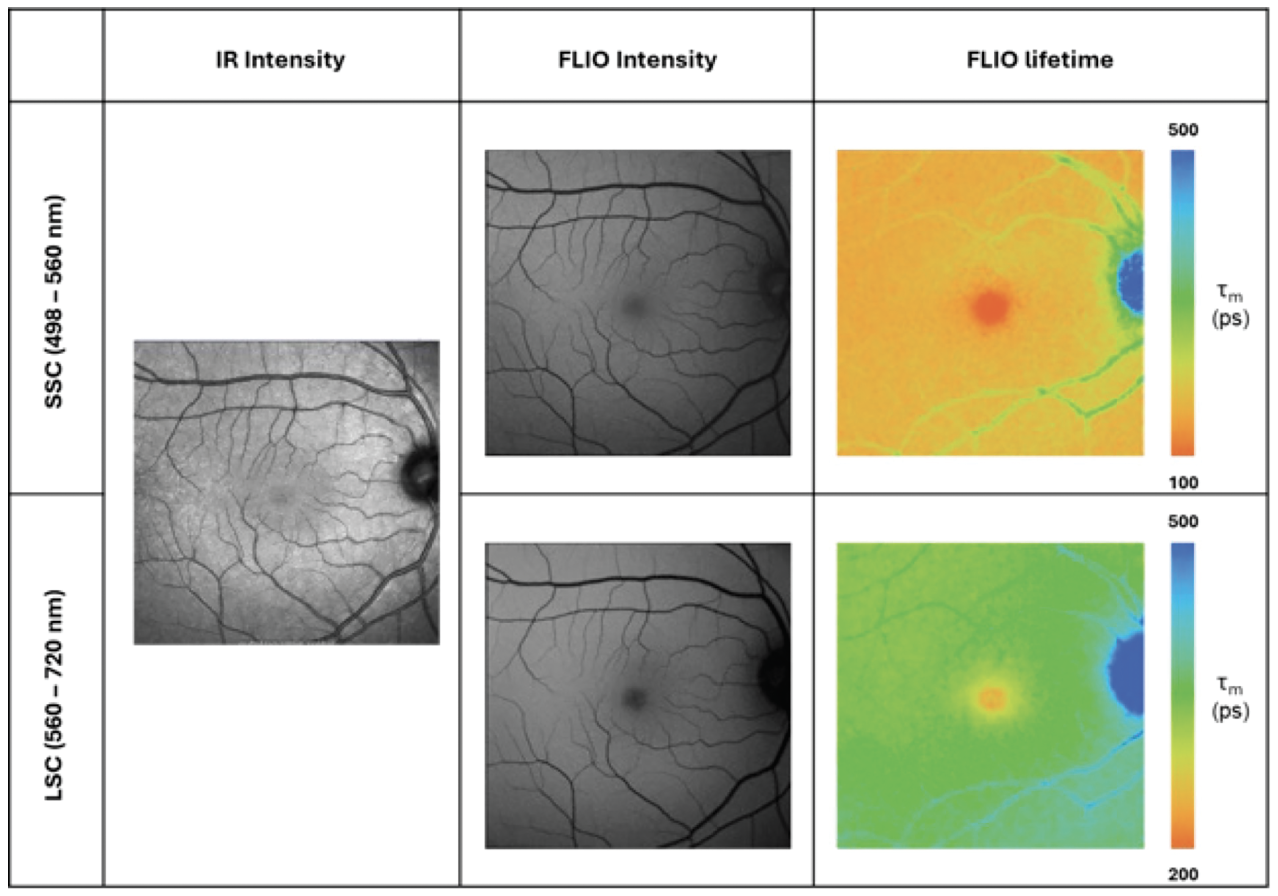
Figure 1. Fundus images captured from healthy eye in clinical-scale FLIO. The panel on the left shows the infrared (IR) intensity image. The panel on the center and right shows the FLIO intensity and the corresponding lifetime variations across the retina in long spectral channels (LSC) and the short spectral channel (SSC) with a blue light excitation. (Captured in collaboration with Paul S. Bernstein at the Moran Eye Center by Janet A.H. Tang). |
Although the current clinical-scale FLIO shows promise as a functional imaging modality that might detect changes at early stages, one of its current limitations is the lack of a simplified metric analogous to RNFL thickness used in OCT for tracking disease progression. A simplified metric or analysis methodology not only makes it easy to use but will generate interest for wide usage in clinical settings.
Clinical-scale FLIO captures fluorescence originating throughout the entire thickness of the retina. It also picks up fluorescence from other parts of the eye like the lens. This makes it difficult to isolate signals specifically from individual retinal layers. To better understand the mechanisms of retinal diseases and their progression, imaging with cellular resolution and isolation to specific retinal layers of interest in living eyes is needed.
Using adaptive optics for in vivo cellular-scale imaging of the RPE mosaic
Thanks to adaptive optics technology, we can non-invasively image the cellular RPE mosaic in people. This technique corrects for the eye’s natural blurring (aberrations), allowing for incredibly sharp pictures of individual retinal cells and targeting of specific cell layers within the retina. AO combined with fluorescence lifetime imaging ophthalmoscopy (AOFLIO) could provide detailed information about the composition and function of cell layers. This approach has been successfully demonstrated in mice,9 monkeys10 and even humans11 using a variety of fluorescence excitation and emission combinations.
For every single pixel in the clinical FLIO image, AOFLIO captures 2,742 pixels of information, allowing finer details to be appreciated. AOFLIO has the potential to provide insight into in vivo cellular-scale changes with age and eccentricity.12 Additionally, comparing the lifetime data from two or more wavelengths of excitation, we can probe into various types of molecules present and how much each contributes. In the eye, lipofuscin, melanin and melanolipofuscin are all naturally occurring and can be distinguished based on the colors of light they emit. An example of fluorescence lifetime using near-infrared (NIR) excitation light can be seen in Figure 2.
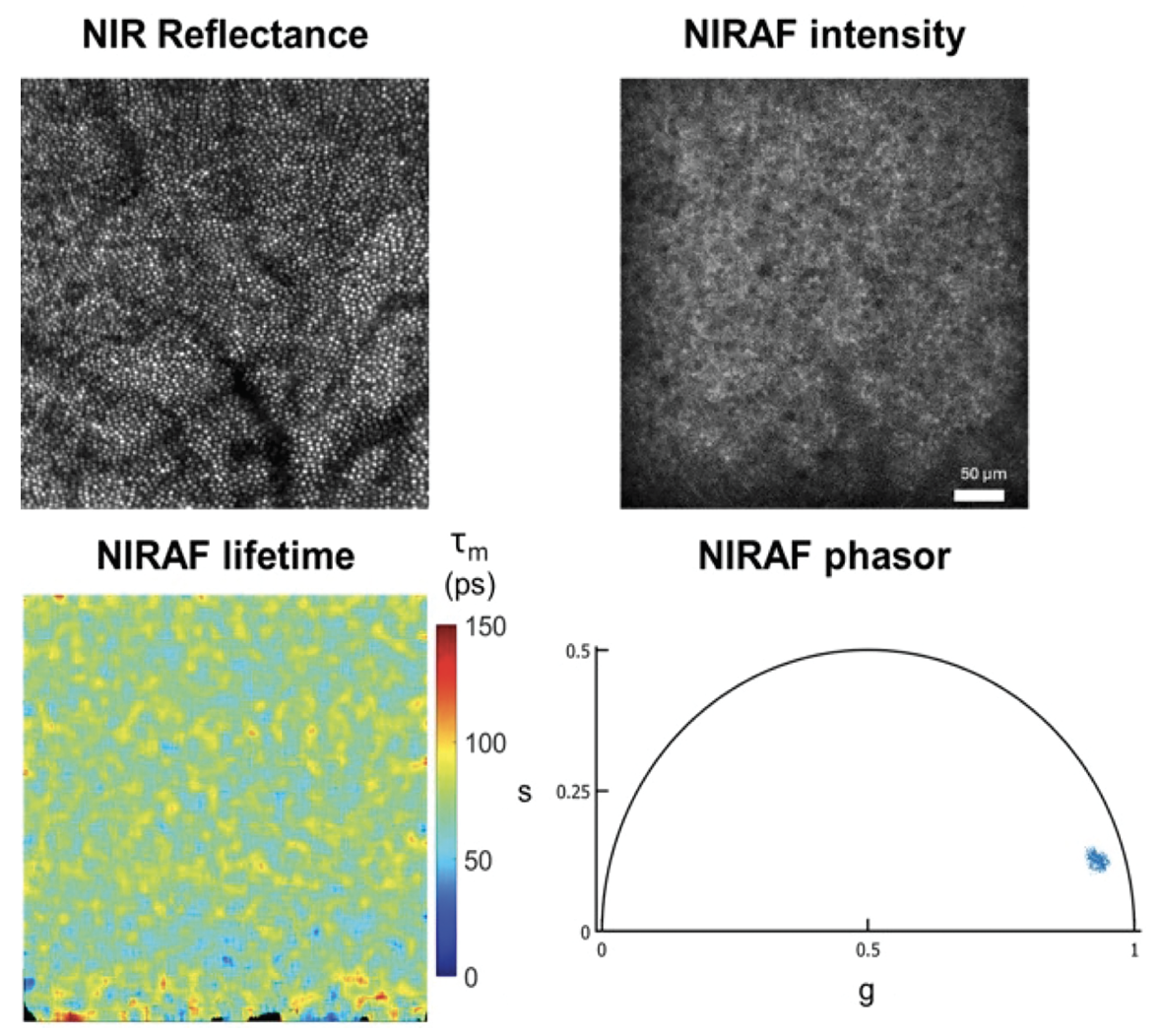 |
| Figure 2. AOFLIO image of the cone photoreceptors at 2 degrees temporal and the corresponding NIR autofluorescence (AF) intensity, mean lifetime and the phasor plot from the RPE in a 30-year-old healthy eye. |
Such pictures can be difficult to interpret so analyzing the lifetime data in a different coordinate space can be sometimes useful in detecting variation. Phasor analysis (a 2D representation of the fluorescence timing in frequency space) is a graphical approach to visualize changes in fluorescence lifetime. Each pixel in the image has a corresponding data point in the phasor plot.13 Phasor analysis in AOFLIO has been successful in differentiating S, M/L, and rod photoreceptors in monkeys and was sensitive enough to capture the functional changes with S cone photodamage.14 Metrics based on phasor analysis have the potential to provide a rapid assessment for comparison between healthy and diseased eyes that will be easily interpreted by clinicians.
Future directions
AOFLIO and FLIO hold promise as a new, non-invasive potential method for better understanding the molecular changes behind cellular variations in retinal diseases. With AOFLIO, we can now compare fluorescence lifetime of the RPE mosaic in living humans at the cellular level and across multiple wavelengths of light, which paves the way for future longitudinal studies. The improved axial resolution afforded by AOFLIO will enable insight into the sources of fluorescence lifetime variations observed with clinical-scale FLIO. FLIO is an easy-to-use imaging method that provides novel information about the sources of retinal fluorescence that are altered in disease. Upcoming investigations using AOFLIO will investigate the cellular mechanisms underlying disease, evaluate the effectiveness of treatments, and ultimately translate these findings into improved patient care. RS
REFERENCES
1. Delori F, Greenberg JP, Woods RL, Fischer J, Duncker T, Sparrow J, Smith RT. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest. Ophthalmol. Vis. Sci. 2011;52:13:9379-9390.
2. Keilhauer CN, Delori FC. Near-infrared autofluorescence imaging of the fundus: Visualization of ocular melanin. Invest. Ophthalmol. Vis. Sci. 2006;47:8:3556-3564.
3. Schweitzer D, Kolb A, Hammer M, et al. Zeitaufgelöste messung der autofluoreszenz. Ophthalmologe. 2002;99:774–779.
4. Dysli C, Wolf S, Berezin MY, Sauer L, Hammer M, Zinkernagel MS. Fluorescence lifetime imaging ophthalmoscopy. Progress in Retinal and Eye Research 2017;60:120-143.
5. Sauer L, Vitale AS, Milliken CM, Modersitzki NK, Blount JD, Bernstein PS. Autofluorescence lifetimes measured with fluorescence lifetime imaging ophthalmoscopy (FLIO) are affected by age, but not by pigmentation or gender. Trans. Vis. Sci. Tech. 2020;9:9:2.
6. Sauer L, Vitale AS, Andersen KM, Hart B, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy (FLIO) patterns in clinically unaffected children of macular telangiectasia type 2 (MACTEL) patients. Retina 2020;40:4:695-704.
7. Sauer L, Gensure RH, Andersen KM, Kreilkamp L, Hageman GS, Hammer M, Bernstein PS. Patterns of fundus autofluorescence lifetimes in eyes of individuals with nonexudative age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2018;59:4:AMD65-AMD77.
8. Fantaguzzi F, Tombolini B, Servillo A, et al. Shedding light on photobiomodulation therapy for age-related macular degeneration: A narrative review. Ophthalmol Ther 2023;12:2903–2915.
9. Feeks JA, Hunter JJ. Adaptive optics two-photon excited fluorescence lifetime imaging ophthalmoscopy of exogenous fluorophores in mice. Biomed Opt Express. 2017;8:2483-2495.
10. Walters S, Feeks JA, Huynh KT, Hunter JJ. Adaptive optics two-photon excited fluorescence lifetime imaging ophthalmoscopy of photoreceptors and retinal pigment epithelium in the living non-human primate eye. Biomed. Opt. Express 2022;13:389-407.
11. Tang JAH, Granger CE, Kunala K, Parkins K, Huynh KT, Bowles-Johnson K, Yang Q, Hunter JJ. Adaptive optics fluorescence lifetime imaging ophthalmoscopy of in vivo human retinal pigment epithelium. Biomed. Opt. Express 2022;13:1737-1754.
12. Kunala K, Tang JAH, Bowles-Johnson K, Huynh KT, Parkins K, Yang Q, JJ Hunter. Near-infrared adaptive optics fluorescence lifetime ophthalmoscopy of human retinal pigment epithelium reveals variations with eccentricity and disease. Invest. Ophthalmol. Vis. Sci. 2023;64:8:467.
13. Stringari C, et al. Metabolic trajectory of cellular differentiation in small intestine by phasor fluorescence lifetime microscopy of NADH. Sci. Rep. 2012;2:568.
14. Huynh KT, Walters S, Foley EK, et al. Separate lifetime signatures of macaque S cones, M/L cones, and rods observed with adaptive optics fluorescence lifetime ophthalmoscopy. Sci Rep. 2023;13:2456.