 |
|
Bio Dr. Xia is a uveitis fellow at the University of Colorado Anschutz Medical Campus. Dr. Hassman is an assistant professor of ophthalmology at the University of Colorado. |
Placoid chorioretinitis refers to a group of inflammatory clinical conditions that have some signs and symptoms in common, but also have unique aspects as well. Discerning these differences is key to a proper diagnosis. Here, we’ll describe the different conditions and how to tell them apart.
The spectrum of conditions
The conditions that make up the spectrum of placoid chorioretinitis share a common characteristic of having focal areas of retinal plaques that typically involve the outer retina and retinal pigment epithelium as well as the underlying choriocapillaris. These conditions include acute posterior multifocal placoid pigment epitheliopathy (APMPPE), persistent placoid maculopathy (PPM) or macular serpiginous choroiditis, relentless placoid chorioretinitis (RPC) or ampiginous chorioretinitis; serpiginous choroiditis (SC), serpiginous-like choroiditis (SLC) associated with tuberculosis, and acute syphilitic posterior placoid chorioretinitis (ASPPC).
These conditions can be associated with infectious or autoimmune diseases or occur without systemic disease. The predominant mechanism is thought to be inflammation that causes hypoperfusion of the choriocapillaris resulting in ischemia to the overlying RPE and outer retina with eventual chorioretinal atrophy.1
|
Key treatment considerations for placoid chorioretinitis:
|
Patients typically present with acute painless blurred vision, photopsia, metamorphopsia, scotomas or floaters. Patients may sometimes have a viral prodrome in APMPPE, RPC or PPM. They can also rarely have neurologic symptoms such as a headache which should raise suspicion for cerebral vasculitis that can be associated with APMPPE, PPM or RPC. They may also have a skin rash or genital lesions consistent with syphilis, and asking about sexual or travel history and sick contacts may help elucidate risk factors for syphilis or TB.
Clinical findings
The size and distribution of the placoid lesions can often help differentiate between the different entities. APMPPE typically manifests as larger (1 to 2-disc areas) creamy white lesions at the level of the RPE that are limited to the posterior pole, while RPC can involve smaller lesions (about half a disc area in size) that become greater in number with disease progression and can spread to involve the periphery.2 PPM typically presents as bilateral, foveal-centered lesions.3 Lesions in SC appear as gray-white lesions that project in a continuous geographic “serpentine” manner from the optic nerve (Figure 1A).4 TB-associated SLC lesions, on the other hand, can involve the posterior pole and periphery and may not involve the peripapillary area until late in the disease. Additionally, serpiginous-like choroiditis lesions can be multifocal as opposed to spreading continuously, like in SC, and are usually accompanied by more significant anterior or vitreous inflammation compared to SC.5,6 Acute syphilitic posterior placoid chorioretinitis typically presents as a subtle yellow whitening of the macula with marked inflammation of the vitreous and anterior chamber (Figure 2A)7 unless the patient is immune-compromised.
The clinical course is also important in differentiating the various placoid conditions. APMPPE is usually acute, monophasic and self-limited. PPM may also present acutely but usually progresses to foveal atrophy without treatment and has higher rates of choroidal neovascularization.8 RPC, on the other hand, has an aggressive clinical course with long periods of disease activity, can relapse over months to years and requires treatment (Figure 3). SC may be initially symptomatic if the lesion starts outside the macula but is a progressive condition that also requires long-term therapy.
Imaging findings
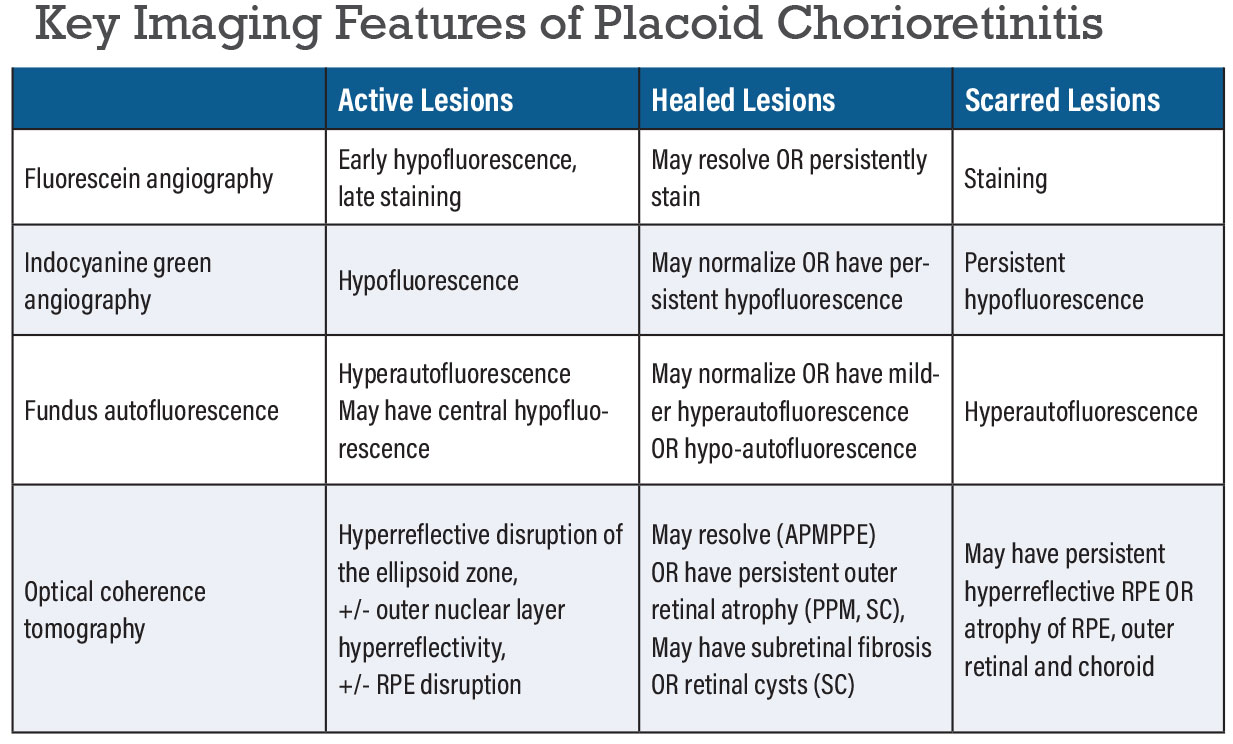
Multimodal imaging can be useful for detecting more lesions than may be clinically apparent and can also help evaluate disease activity. The different placoid entities share some common imaging features that can help differentiate them from other inflammatory chorioretinal disorders. Fundus autofluorescence can show hyperautofluorescence at the edges of active lesions (Figure 3B) that can also have hypoautofluorescent halos in SC (Figure 1B).9,10 Placoid lesions on FAF will generally transition from hyperAF to hypoAF once lesions become inactive, however areas of RPE hyperplasia or scarring may remain hyperAF. Fluorescein angiography shows early hypofluorescence and late hyperfluorescence of the lesions, especially at the edge of an active lesion (Figure 1C) or in areas of scar or chorioretinal atrophy.11
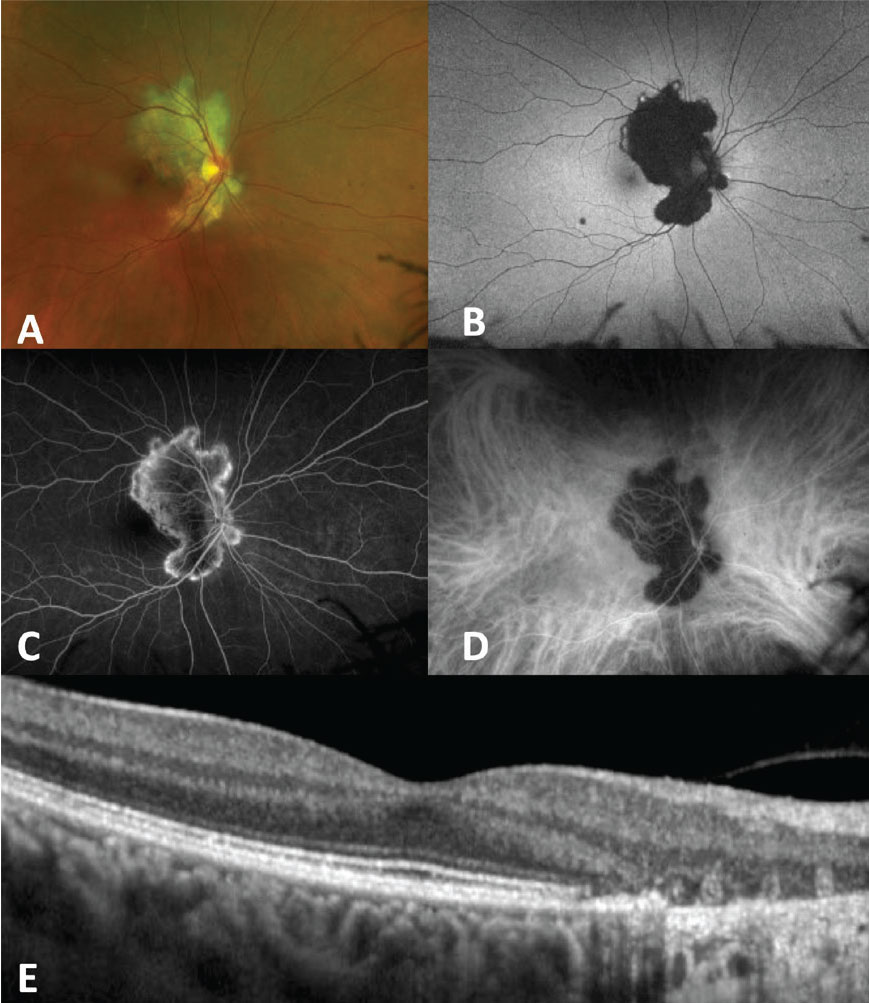 |
| Figure 1. Serpiginous choroiditis. A) Fundus photo shows a gray-white geographic lesion extending from the optic nerve. B) Fundus autofluorescence shows hyperautofluorescence at active areas of the lesion with hypoautofluorescent center where disease is currently inactive. C) Fluorescein angiography shows late hyperfluorescence of lesion edges. D) Indocyanine green angiography shows hypoautofluorescence throughout the lesion. E) Optical coherence tomography shows disruption of the ellipsoid zone and outer retina, as well as atrophy of the retinal pigment epithelium. |
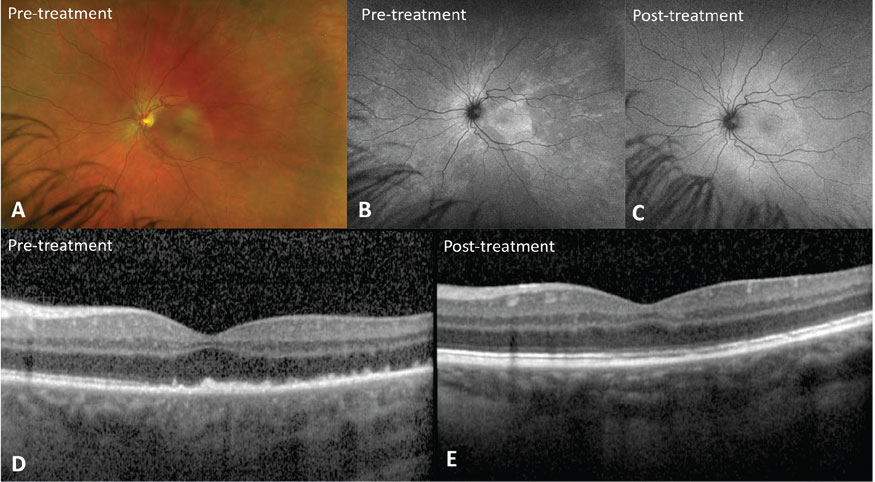 |
| Figure 2. Acute syphilitic posterior placoid chorioretinitis. A) Fundus photo shows a yellow placoid lesion in the macula. B) Fundus autofluorescence demonstrates hyperautofluorescence corresponding to the placoid lesion that resolves after treatment with intravenous penicillin (C). D) Optical coherence tomography shows punctate spots in the choroid, ellipsoid zone disruption, and a nodular appearance of the retinal pigment epithelium that are restored after treatment (E). |
OCTA demonstrates hyporeflective areas of perfusion deficits at the level of the choriocapillaris in active disease.18-23 OCTA studies suggest that FAF lesions may lag behind choriocapillary flow voids seen on OCTA.19 Indocyanine green angiography shows early and late hypofluorescence corresponding to placoid lesions due to choroidal ischemia (Figure 1D) which may partially or completely resolve or persist with disease inactivity.12,13
Optical coherence tomography through active placoid lesions shows increased choroidal thickness, hyperreflective disruption of the ellipsoid zone in all cases and variably of the outer nuclear layer and retinal pigment epithelium. Ultimately, there can be recovery of the outer retinal layers in conditions like APMPPE or syphilitic chorioretinitis after treatment. However, in conditions like SLC and RPC, placoid lesions tend to transition to outer retinal atrophy and photoreceptor loss in the inactive stages of the disease (Figure 1E).9,14-16 The OCT findings that are specific to ASPPC include punctate hyper-reflective spots in the choroid and nodular appearing RPE (Figure 2D).17
 |
 |
|
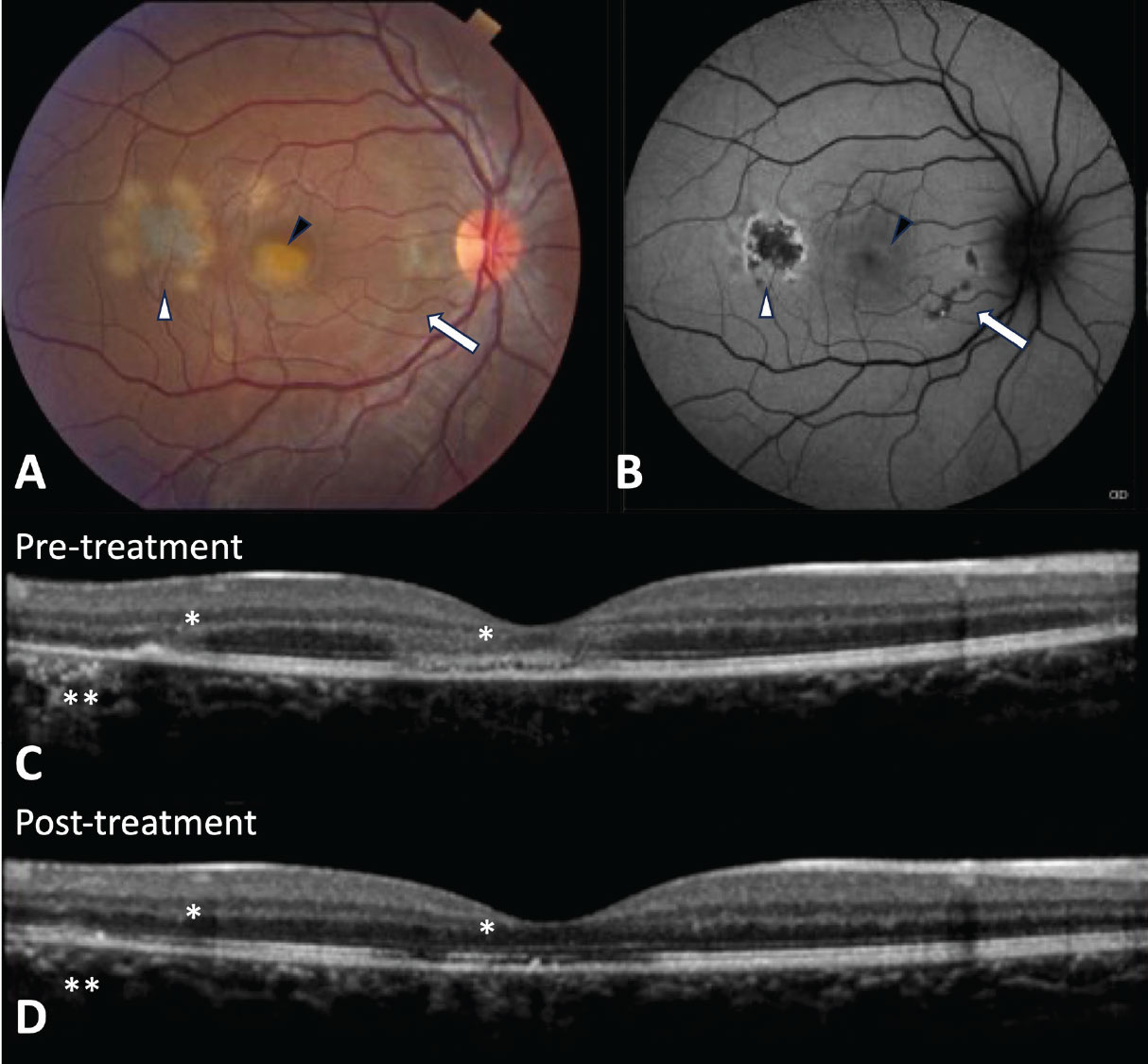
Figure 3. Relentless placoid chorioretinitis (RPC). A) Fundus photo shows creamy yellow/white lesions at the level of the retinal pigment epithelium in the posterior pole that was initially diagnosed as acute posterior multifocal placoid pigment epitheliopathy. However, this patient later returned with new lesions suggesting a diagnosis of RPC. B) Fundus autofluorescence shows a new active lesion in the temporal macula with hyperautofluorescent edges (white arrowhead) and fovea with subtle hyperautoflouresence (black arrowhead) and healed lesions (arrow) that are hypoautoflourescent and minimally apparent on the fundus photo. C) Optical coherence tomography shows hyperreflective disruption of the ellipsoid zone and outer nuclear layer (asterisk) as well as attenuation and hyperreflectivity of the retinal pigment epithelium (double asterisk) that partially recover after treatment with immunosuppression (D). |
Diagnosis
Diagnostic workup to differentiate between infectious and autoimmune etiologies is imperative to determine the correct therapeutic approach. Infectious causes of placoid chorioretinitis include primarily syphilis, TB and possibly coxsackievirus. Less common pathogens include Histoplasma, Group A streptococcus, adenovirus type 5 and Borrelia (Lyme disease). Autoimmune diseases associated with placoid chorioretinitis can include sarcoidosis (Figure 4), cerebral vasculitis, ulcerative colitis, granulomatosis and polyangiitis. Labs should, at a minimum, include RPR, QuantiFERON Gold and chest X-ray. Clinical evaluation should include a careful review of systems for further targeted testing or subspecialist referral. Any patient with placoid lesions and neurological symptoms, including new or unusual headaches, should undergo urgent neuroimaging to evaluate for cerebral vasculitis that can be associated with
APMPPE, PPM and RPC.
 |
|
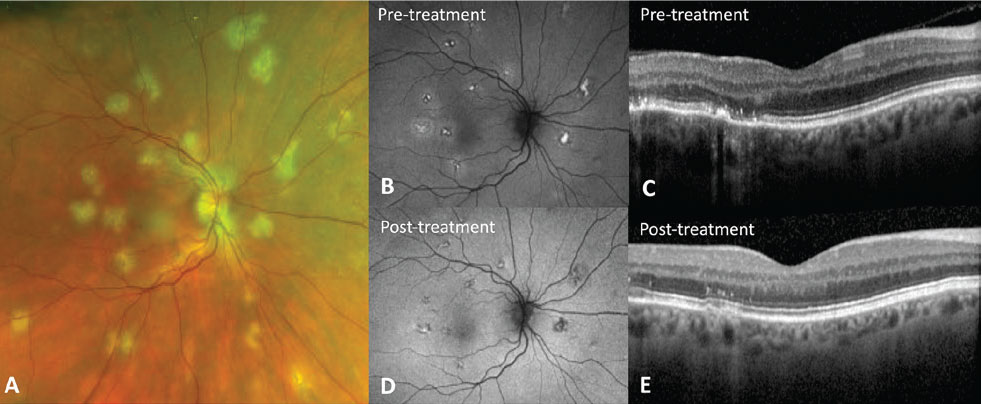
Figure 4. Placoid chorioretinitis associated with neurosarcoidosis. A) Fundus photo shows multiple creamy white placoid lesions in the posterior pole. B) Fundus autofluorescence shows mixed hypo and hyperfluorescent areas corresponding to the placoid lesions. C) Optical coherence tomography shows hyperreflective disruption of the ellipsoid zone, outer nuclear layer and retinal pigment epitheliaum. D) Fundus autofluorescence shows decreased hyperautofluorescence with restoration of the ellipsoid zone on OCT (E) after treatment with intravitreal steroid and systemic immunosuppression. |
Treatment
Treatment of infectious placoid chorioretinitis is targeted at the underlying infection with generally good resolution and visual outcomes afterward. Syphilitic chorioretinitis requires treatment with 10 to 14 days of intravenous penicillin, which leads to the resolution of abnormal autofluorescence (Figure 2C) and restoration of the RPE and outer retina on OCT (Figure 2E).17 Placoid chorioretinitis associated with TB or pulmonary histoplasmosis requires anti-TB and anti-fungal therapy, respectively, and may also require adjunctive steroid therapy to control inflammation.
Self-limiting conditions like APMPPE may not always require treatment,24 but vision-threatening lesions should be treated. Outside of APMPPEE, other forms of non-infectious placoid chorioretinitis are usually aggressive, requiring immunosuppression with steroid therapy first, which can be in the form of systemic steroids or local injections. Patients with recurrent disease are then escalated to steroid-sparing immunosuppression.
Outcomes
Visual outcomes are quite favorable, as outer retinal irregularities improve with treatment (Figures 3 and 4), but final visual acuity also depends on the location and degree of retinal atrophy that can remain after disease activity subsides.24 Complications such as choroidal neovascularization are higher in PPM and SC than in other types of placoid chorioretinitis and may warrant anti-VEGF therapy.25,26
Bottom Line
Placoid chorioretinitis can have many etiologies, ranging from infectious to autoimmune. Clinical findings, disease course and laboratory work-up can help differentiate between different placoid entities. Treatment of infectious placoid conditions are targeted at the underlying infection while non-infectious etiologies can require a combination of steroid and immunosuppressive agents. RS
REFERENCES
1. Deutman AF, Lion F. Choriocapillaris nonperfusion in acute multifocal placoid pigment epitheliopathy. Am J Ophthalmol. Nov 1977;84:5:652-7.
2. Mirza RG, Jampol LM. Relentless placoid chorioretinitis. Int Ophthalmol Clin. Fall 2012;52:4:237-42.
3. Kolomeyer AM, Brucker AJ. Persistent placoid maculopathy: a systematic review. Retina. Oct 2018;38:10:1881-1895.
4. Lim WK, Buggage RR, Nussenblatt RB. Serpiginous choroiditis. Surv Ophthalmol. May-Jun 2005;50:3:231-44.
5. Nazari Khanamiri H, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. May-Jun 2013;58:3:203-32.
6. Gupta V, Shoughy SS, Mahajan S, et al. Clinics of ocular tuberculosis. Ocul Immunol Inflamm. Feb 2015;23:1:14-24.
7. Eandi CM, Neri P, Adelman RA, Yannuzzi LA, Cunningham ET Jr, International Syphilis Study G. Acute syphilitic posterior placoid chorioretinitis: report of a case series and comprehensive review of the literature. Retina. Oct 2012;32:9:1915-41.
8. Gendy MG, Fawzi AA, Wendel RT, Pieramici DJ, Miller JA, Jampol LM. Multimodal imaging in persistent placoid maculopathy. JAMA Ophthalmol. Jan 2014;132:1:38-49.
9. Arantes TE, Matos K, Garcia CR, Silva TG, Sabrosa AS, Muccioli C. Fundus autofluorescence and spectral domain optical coherence tomography in recurrent serpiginous choroiditis: case report. Ocul Immunol Inflamm. Feb 2011;19:1:39-41.
10. Cardillo Piccolino F, Grosso A, Savini E. Fundus autofluorescence in serpiginous choroiditis. Graefes Arch Clin Exp Ophthalmol. Feb 2009;247:2:179-85.
11. Marchese A, Agarwal AK, Erba S, et al. Placoid lesions of the retina: progress in multimodal imaging and clinical perspective. Br J Ophthalmol. Jan 2022;106:1:14-25.
12. Dhaliwal RS, Maguire AM, Flower RW, Arribas NP. Acute posterior multifocal placoid pigment epitheliopathy: an indocyanine green angiographic study. Retina. 1993;13:4:317-25.
13. Agrawal RV, Biswas J, Gunasekaran D. Indocyanine green angiography in posterior uveitis. Indian J Ophthalmol. Apr 2013;61:4:148-59.
14. Goldenberg D, Habot-Wilner Z, Loewenstein A, Goldstein M. Spectral domain optical coherence tomography classification of acute posterior multifocal placoid pigment epitheliopathy. Retina. Jul 2012;32:7:1403-10.
15. van Velthoven ME, Ongkosuwito JV, Verbraak FD, Schlingemann RO, de Smet MD. Combined en-face optical coherence tomography and confocal ophthalmoscopy findings in active multifocal and serpiginous chorioretinitis. Am J Ophthalmol. May 2006;141:5:972-5.
16. Bansal R, Kulkarni P, Gupta A, Gupta V, Dogra MR. High-resolution spectral domain optical coherence tomography and fundus autofluorescence correlation in tubercular serpiginouslike choroiditis. J Ophthalmic Inflamm Infect. Dec 2011;1:4:157-63.
17. Pichi F, Ciardella AP, Cunningham ET Jr, et al. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina. Feb 2014;34:2:373-84.
18. Mangeon M, Zett C, Amaral C, et al. Multimodal evaluation of patients with acute posterior multifocal placoid pigment epitheliopathy and serpiginous choroiditis. Ocul Immunol Inflamm. 2018;26:8:1212-1218.
19. Pakzad-Vaezi K, Khaksari K, Chu Z, Van Gelder RN, Wang RK, Pepple KL. Swept-source OCT angiography of serpiginous choroiditis. Ophthalmol Retina. Jul 2018;2:7:712-719.
20. Montorio D, Giuffre C, Miserocchi E, et al. Swept-source optical coherence tomography angiography in serpiginous choroiditis. Br J Ophthalmol. Jul 2018;102:7:991-995.
21. Park SS, Thinda S, Kim DY, Zawadzki RJ, Werner JS. Phase-variance optical coherence tomographic angiography imaging of choroidal perfusion changes associated with acute posterior multifocal placoid pigment epitheliopathy. JAMA Ophthalmol. Aug 1 2016;134:8:943-5.
22. Klufas MA, Phasukkijwatana N, Iafe NA, et al. Optical coherence tomography angiography reveals choriocapillaris flow reduction in placoid chorioretinitis. Ophthalmol Retina. Jan-Feb 2017;1:1:77-91.
23. Heiferman MJ, Rahmani S, Jampol LM, Nesper PL, Skondra D, Kim LA, Fawzi AA. Acute posterior multifocal placoid pigment epitheliopathy on optical coherence tomography angiography. Retina. Nov 2017;37:11:2084-2094.
24. Fiore T, Iaccheri B, Androudi S, et al. Acute posterior multifocal placoid pigment epitheliopathy: outcome and visual prognosis. Retina. Jul-Aug 2009;29:7:994-1001.
25. Parodi MB, Iacono P, Bandello F. Juxtafoveal choroidal neovascularization secondary to persistent placoid maculopathy treated with intravitreal bevacizumab. Ocul Immunol Inflamm. Oct 2010;18:5:399-401.
26. Waisbren EC, Ho J, Smithen LM, Yannuzzi LA, Duker JS. Using ranibizumab to successfully treat choroidal neovascularization in a patient with persistent placoid maculopathy. Retin Cases Brief Rep. Summer 2010;4:3:211-5.




