Take-home points
|
 |
Bios Dr. Bommakanti is a vitreoretinal surgery fellow at Wills Eye Hospital/Mid Atlantic Retina, Philadelphia. Dr. Chiang is an attending at Wills Eye Hospital/Mid Atlantic Retina and Associate Clinical Professor at Thomas Jefferson University, Philadelphia. Dr. Bommakanti disclosed financial relationships with RegenXbio and Alimera Sciences. Dr. Chiang disclosed financial relationships with Apellis Pharmaceuticals, Genentech/Roche and Gyroscope Therapeutics. |
Imaging is becoming an increasingly important aspect of managing age-related macular degeneration in general, and geographic atrophy in particular, with many studies prioritizing GA growth on imaging over visual function in terms of endpoints. Here, we’ll review recent expert recommendations for the assessment of geographic atrophy using various imaging modalities.
The GA Landscape
Geographic atrophy is a late form of age-related macular degeneration that’s characterized by complete loss of the retinal pigment epithelium and outer retina of least 250 µm in diameter in the absence of macular neovascularization.1,2 Dysregulation of the complement pathway is implicated in AMD,3-5 and in 2023 the FDA approved two complement inhibitors, pegcetacoplan injection (Syfovre, Apellis Pharmaceuticals), which binds and inhibits centrally at the level of C3 and C3b and avancincaptad pegol (ACP, Izervay, Iveric Bio), which acts at C5, based on the results of the OAKS/DERBY6 and GATHER1/GATHER27 clinical trials, respectively.
GA has historically been identified and monitored by funduscopic examination and color fundus photography.8 Advances in retinal imaging, including fundus autofluorescence (FAF), near-infrared reflectance (NIR), spectral-domain optical coherence tomography (SD-OCT) imaging have revolutionized the ability to identify and monitor GA. In fact, as alluded to earlier, the primary endpoints of OAKS, DERBY, GATHER1, and GATHER2 relied on imaging, rather than visual acuity, endpoints.6,7 GA imposes a significant burden on patients and, considering the recently FDA approved therapies, there’s increased interest in the management of GA.
An international group of experts, the Classification of Atrophy Meeting (CAM) Group, has produced several important reports, including consensus guidelines for imaging protocols in AMD studies,9 definitions of atrophy on OCT,2,10 and descriptions of OCT features associated with progression to GA.11 The group’s recommendations are reviewed below, in the order that CAM addressed them in its report.
Color fundus photography
Color fundus photography (CFP) was used in the initial definition of GA and has historically been used to identify GA in clinical trials.8 GA on CFP is defined as round or oval sharply demarcated areas with a hypopigmented or depigmented appearance and increased visibility of the underlying choroidal vessels (Figure 1A). Advantages of CFP include the large volume of historical images for comparison and the true color rendition of the fundus (the white light captures images that look like just like how the macula appears on ophthalmoscopy).
There are several disadvantages, though. CFP images have low contrast, which can make it difficult to identify the border of a GA lesion. CFP also requires a bright light, which can make the result susceptible to image degradation from media opacities and is uncomfortable for patients. The CAM group recommends obtaining CFP at the baseline and final visits for clinical trials.9 In practice, we often obtain CFP at baseline, but mainly rely on other modalities at subsequent visits.
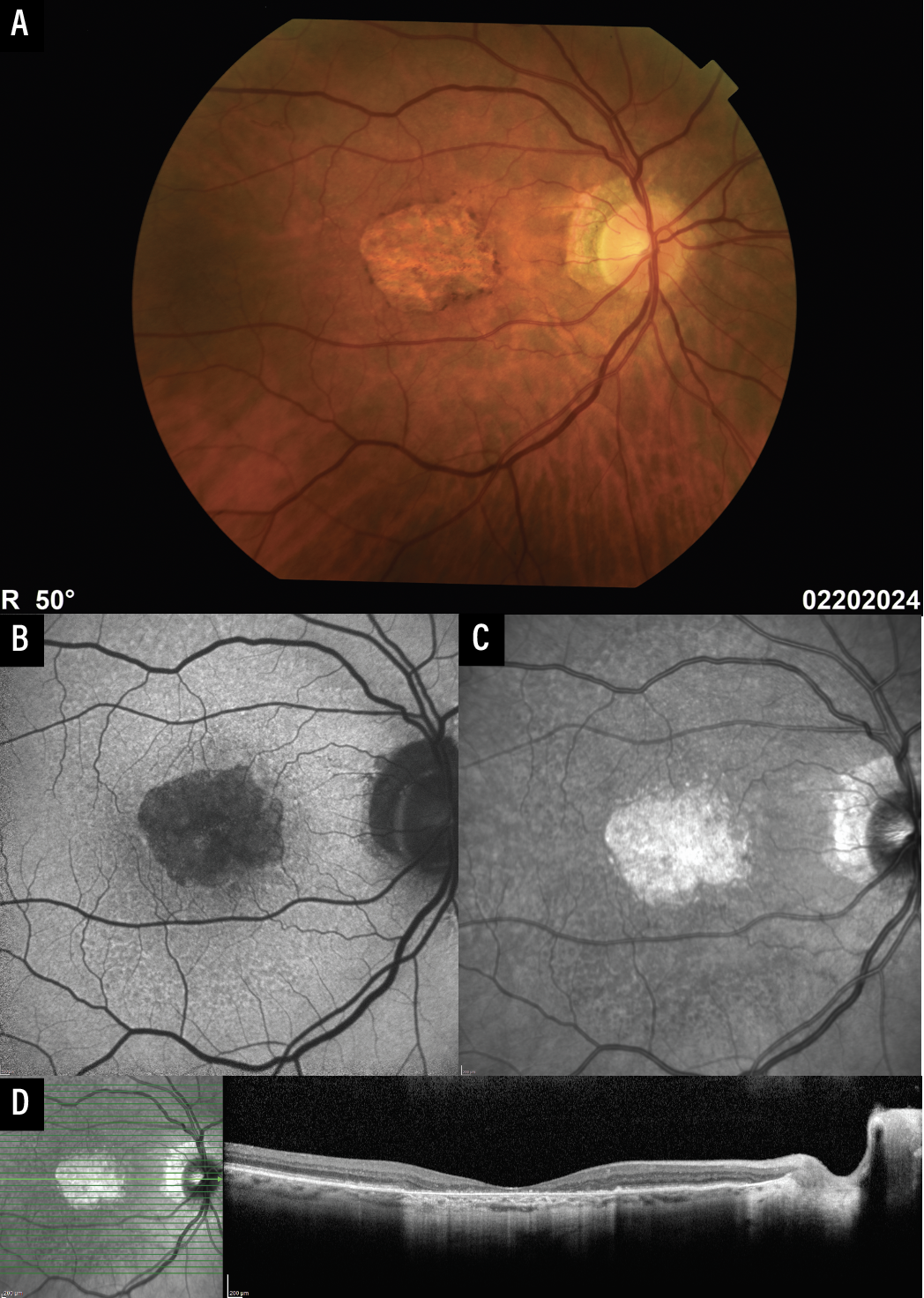 |
| Figure 1. Multimodal imaging for geographic atrophy. Color fundus photography (A), fundus autofluorescence (B), near-infrared reflectance imaging (C), and optical coherence tomography (D). |
Fundus autofluorescence
Fundus autofluorescence captures emission by intrinsic fluorophores, predominantly lipofuscin in the RPE,12 and is a primary endpoint for clinical trials.6,7,13 GA is hypoautofluorescent on FAF due to loss of the RPE, and FAF often provides a clear demarcation between atrophic and non-atrophic regions (Figure 1B). Most devices use blue light for excitation, which can result in difficulty identifying GA that involves the fovea since the fovea in normal eyes is already hypoautofluorescent due to central macular pigments that absorb blue light (Figure 2A).9 Green light excitation FAF is available and can mitigate this problem, however supplementing FAF images with near-infrared reflectance images (see below) is also feasible and is more commonly done.
FAF uses a bright light that can be uncomfortable to patients and result in interference by media opacities, but in general it’s well-tolerated, acquisition isn’t difficult, and most OCT platforms and/or fundus cameras have the option of FAF capability. Given that it’s a primary study endpoint used to delineate GA size and lesion characteristics, we periodically obtain FAF at least once or twice a year, particularly in patients undergoing treatment for GA.
Near-infrared reflectance
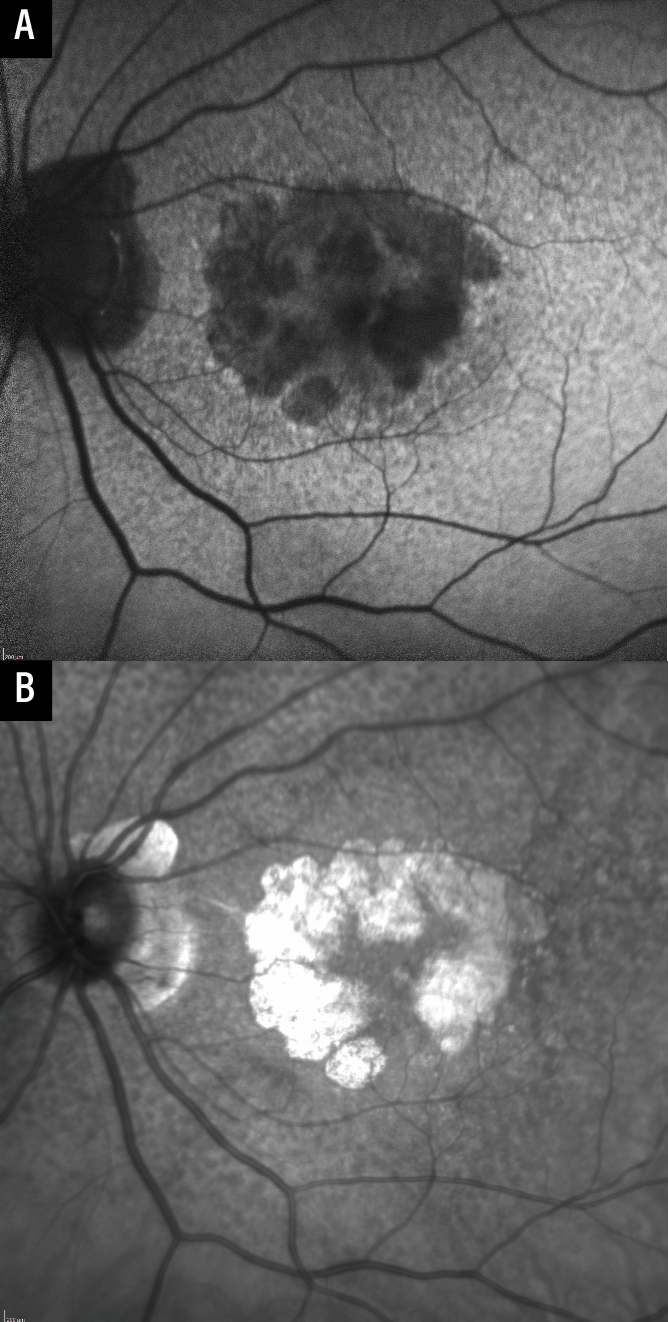 |
| Figure 2. Near-infrared reflectance imaging as an adjunct to fundus autofluorescence. The fovea appears hypofluorescent on fundus autofluorescence as blue light is absorbed by macular pigments (A). Near-infrared reflectance imaging can be used to determine the presence or absence of fovea-involving geographic atrophy (B). |
Near-infrared reflectance imaging uses light at the higher end of the visible spectrum (750 to 840 nm). It initially started as a guidance modality for OCT, but it’s now available as an independent output for many devices.9 GA appears hyperreflective on NIR, and the borders are often also sharply demarcated, similar to FAF (Figure 1C). There’s no absorption by macular pigments (allowing easier visualization of GA involving the fovea; Figure 2B), lesions that predispose to GA (such as subretinal drusenoid deposits) can be seen well on NIR, and NIR is also comfortable for patients. Although use of NIR alone hasn’t been well validated in studies, we find it to be very useful when obtained and interpreted in conjunction with OCT, particularly in cases where FAF image quality is suboptimal.
Multicolor imaging
Multicolor imaging combines blue (488 nm), green (518 nm) and near-infrared (820 nm) reflectance images obtained using confocal scanning lasers. Since different wavelengths highlight different details at different depths, this enables good images of the vitreoretinal interface and inner retina (blue), deeper retina (green), and outer retina and choroid (near-infrared) to be obtained. It’s not yet clear, though, if this modality adds any utility beyond NIR so we don’t routinely obtain multicolor images.
Optical coherence tomography
Spectral-domain and swept-source OCT provide high resolution, three-dimensional cross sections through the retina which provide information about the different retinal layers involved in GA. OCT B-scans can also be reconstructed into C-scans, which are en face images that can be selectively analyzed at varying depths within the retina, RPE and choroid.
Recognizing the utility of OCT imaging in this condition and the potential problems associated with the term “GA,”1 the CAM group recommended OCT be used as the reference imaging modality to identify atrophy and proposed “complete RPE and outer retinal atrophy” (cRORA) as a broader term that includes GA as well as macular atrophy with coexisting or prior neovascularization.2 cRORA is defined as 1) hypertransmission of at least 250 µm, 2) RPE attenuation of at least 250 µm, and 3) photoreceptor disruption (i.e., disruption of the IZ, EZ, or ELM, and/or thinning of the ONL) in the absence of an RPE tear, and is identified on OCT B-scans (Figure 1D).2 Although outside the scope of this article, OCT is also useful for identifying “incomplete RPE and outer retinal atrophy” (iRORA), which is nascent GA without MNV,2,10 and lesions that predispose to GA.11
The near-infrared light used to acquire an OCT is comfortable for patients. Eye-tracking software and image registration, which improve colocalization analysis, enable the reliable comparison of scans over time. It takes longer to review OCT images because all B-scans of a volumetric scan or all en face slabs need to be reviewed, although AI-driven software will likely accelerate image processing in the future. OCT is essential in clinical practice, and we obtain OCT for all patients with GA at all visits.
Application to clinical practice and bottom line
The approved therapies for GA are still relatively new, and there are many potential treatments in the research pipeline. Our understanding of GA continues to evolve, and our ability to detect it has improved thanks to advances in imaging.
FAF, NIR and OCT are all noninvasive imaging modalities that provide complementary information about GA. Clinical utility, acquisition time, patient comfort and cost all factor into the clinical decision to obtain any particular imaging test. We recommend an OCT-based strategy (we always obtain NIR with OCT) with periodic FAF when imaging GA. Although CFP has been used for clinical trials, we typically only obtain it at baseline. We don’t obtain multicolor imaging.
Invasive and noninvasive angiography including FA, ICGA and OCTA are useful to either confirm or characterize macular neovascularization but are outside the scope of this article. Improved understanding of the histologic correlates to atrophic and pre-atrophic lesions on imaging as well as advances in technology, such as AI-driven image analysis, will inevitably enhance our ability to identify, monitor and possibly predict GA progression over time. RS
References
1. Schmitz-Valckenberg S, Sadda S, Staurenghi G, Chew EY, Fleckenstein M, Holz FG. Geographic atrophy: Semantic considerations and literature review. Retina. 2016;36:12:2250-2264.
2. Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT. Ophthalmology. 2018;125:4:537-548.
3. Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7:1:31.
4. Yates JRW, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:6:553-561.
5. Boyer DS, Schmidt-Erfurth U, Van Lookeren Campagne M, Henry EC, Brittain C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37:5:819-835.
6. Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. The Lancet. 2023;402:10411:1434-1448.
7. Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. The Lancet. 2023;402:10411:1449-1458.
8. Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Survey of Ophthalmology. 1995;39:5:367-374.
9. Holz FG, Sadda SR, Staurenghi G, et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: Recommendations from classification of atrophy consensus meetings. Ophthalmology. 2017;124:4:464-478.
10. Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration. Ophthalmology. 2020;127:3:394-409.
11. Jaffe GJ, Chakravarthy U, Freund KB, et al. Imaging features associated with progression to geographic atrophy in age-related macular degeneration. Ophthalmology Retina. 2021;5:9:855-867.
12. Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging. Retina. 2008 Mar;28:3:385-409
13. Csaky K, Ferris F, Chew EY, Nair P, Cheetham JK, Duncan JL. Report From the NEI/FDA Endpoints workshop on age-related macular degeneration and inherited retinal diseases. Invest Ophthalmol Vis Sci. 2017;58:9:3456.



