Take-home points
|
 |
Bios Dr. Paschalis Ilios is an assistant professor of ophthalmology at Harvard Medical School (HMS) and serves as the director of Research, Development, and Regulatory Affairs at Boston Keratoprosthesis, Massachusetts Eye and Ear (MEE). Jyoti Sharma is a post-doctoral fellow in Dr. Paschalis’ laboratory at Mass. Eye and Ear, where she focuses on advancing treatments for corneal blindness. |
Ocular diseases, such as uveitis, age-related macular degeneration, diabetic retinopathy and retinal vein occlusion, are leading causes of permanent vision loss worldwide,1-3 and inflammation plays a critical role in the pathophysiology of these conditions, contributing to progressive retinal degeneration. Among the numerous cytokines implicated in ocular inflammation, tumor necrosis factor-alpha, a potent pro-inflammatory cytokine produced by reactive phagocytes and lymphoid cells, has emerged as a key player. This article explores the potential therapeutic role of TNF-α inhibitors in treating retinal diseases.
TNF-α involvement in retinal inflammation
TNF-α exerts its effects by binding to TNF receptors 1 (TNFR1) and 2 (TNFR2). TNFR1 is expressed ubiquitously across mammalian cells, while TNFR2 is primarily expressed in immune, endothelial and neuronal cells. In the retina, TNF-α is produced by a variety of cells, including microglia, infiltrating macrophages/monocytes, Müller cells and the retinal pigment epithelium. This cytokine contributes to tissue inflammation and damage by activating cell-death mechanisms. In tissues with non-proliferative cells, such as the central nervous system, this process leads to gradual neuronal cell loss and subsequent neurodegeneration. TNFR1 activation induces the expression of additional pro-inflammatory cytokines, chemokines and adhesion molecules, and increases vascular permeability, promoting the recruitment of reactive immune cells to the tissue.4,5 Disruption of the blood-retinal barrier leads to neovascularization and further destabilizes retinal homeostasis. Inflammation also mediates ganglion cell loss, which precedes changes in pericytes or acellular capillaries, suggesting that inflammation and cell death independently causes vascular alterations.6,7 Although VEGF inhibitors, currently used to treat retinal neovascular diseases, are effective against neovascularization, they have limited efficacy in suppressing pre-existing tissue inflammation. Consequently, combination therapies targeting both VEGF and TNF-α have been proposed and explored in relevant animal models.8
 |
|
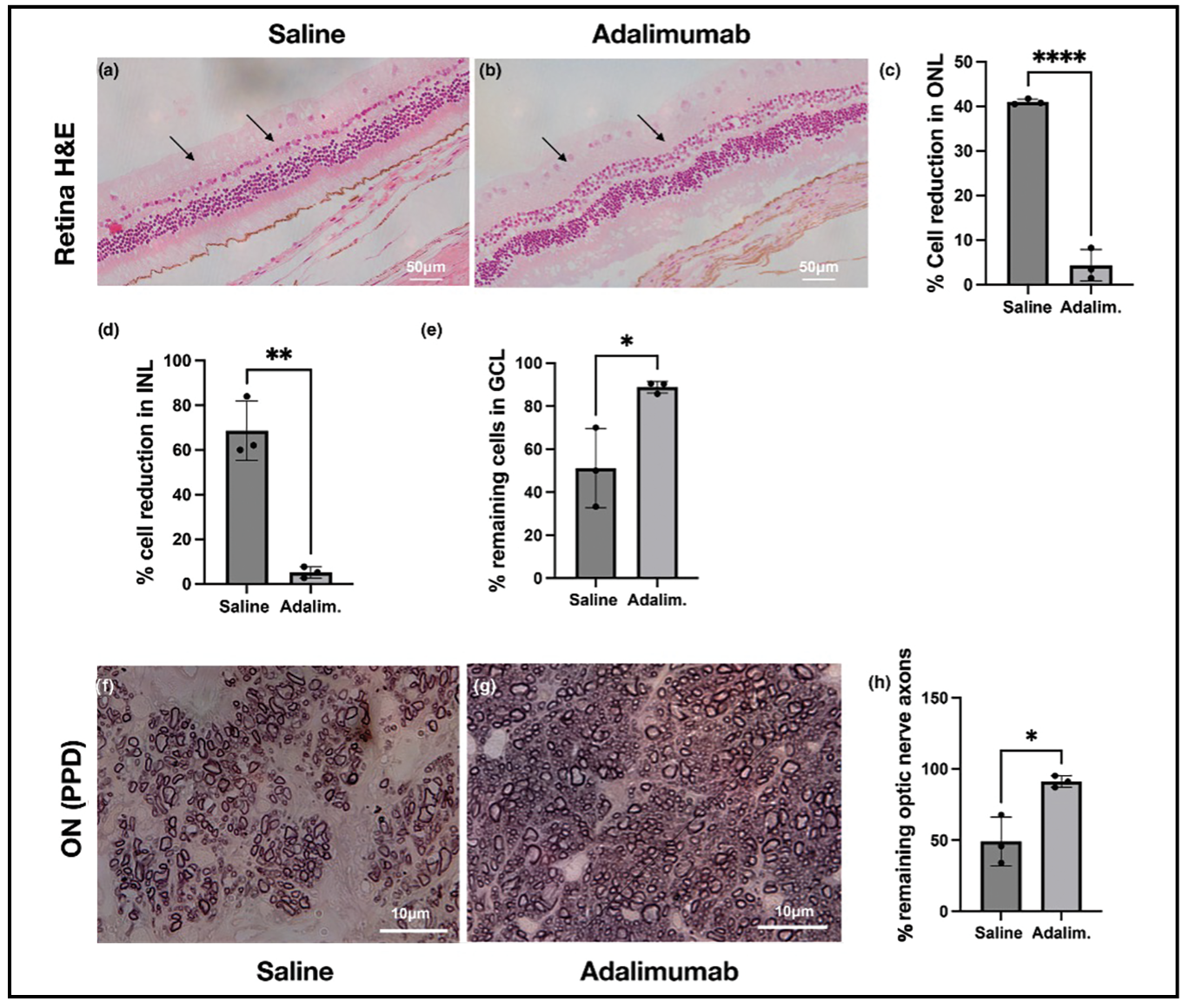
Figure 1. H&E and PPD staining three months after corneal alkali injury and subsequent treatment with a single subconjunctival injection of 4 mg adalimumab demonstrates significant protection to the retina and optic nerve. |
Therapeutic potentials of TNF-α inhibitors
Corticosteroids have long been the cornerstone of therapy for diabetic macular edema, retinal vein occlusion and AMD, effectively stabilizing vessel permeability and reducing exudative processes and macular edema. However, their broad action often leads to significant side effects due to a lack of specificity. This led to the development of monoclonal antibodies (mAbs), such as VEGF inhibitors, to specifically target retinal neovascularization.
Similarly, TNF-α inhibitors have become the gold standard for treating non-infectious uveitis, offering improved efficacy in suppressing inflammation compared to corticosteroids. Recent studies, including our own, have demonstrated that low-dose subconjunctival administration of adalimumab can prevent uveal and retinal inflammation, improving drug bioavailability to the retina by 10 to 100 times compared to systemic administration.9,10 TNF-α inhibitors have also been shown to reduce ocular neovascularization in animal models, suggesting their potential use in treating neovascular diseases. In the context of alkali burns, TNF-α inhibitors were found to prevent secondary retinal ganglion cell loss and optic nerve degeneration (Figure 1), highlighting new prospects for their use in injuries. With the rapid expansion of biosimilars, TNF-α-targeting antibodies have the potential to revolutionize ocular disease therapy, particularly in terms of retinal protection.
Synergistic effect of combination therapy with TNF-α and VEGF Inhibitors
While TNF-α inhibitors can suppress ocular inflammation and neovascularization, some degree of angiogenesis may persist. VEGF inhibitors may be more effective against neovascularization, but they don’t block inflammation, allowing neovascularization to potentially continue. We recently investigated the synergistic effects of concomitant TNF-α and VEGF inhibition, demonstrating that this combination enhances anti-angiogenesis and provides nearly complete blockade of vessel growth in an acute ocular injury model. Moreover, this therapy was able to prevent neuroretinal and optic nerve axon damage (Figure 2), which is clinically very significant.
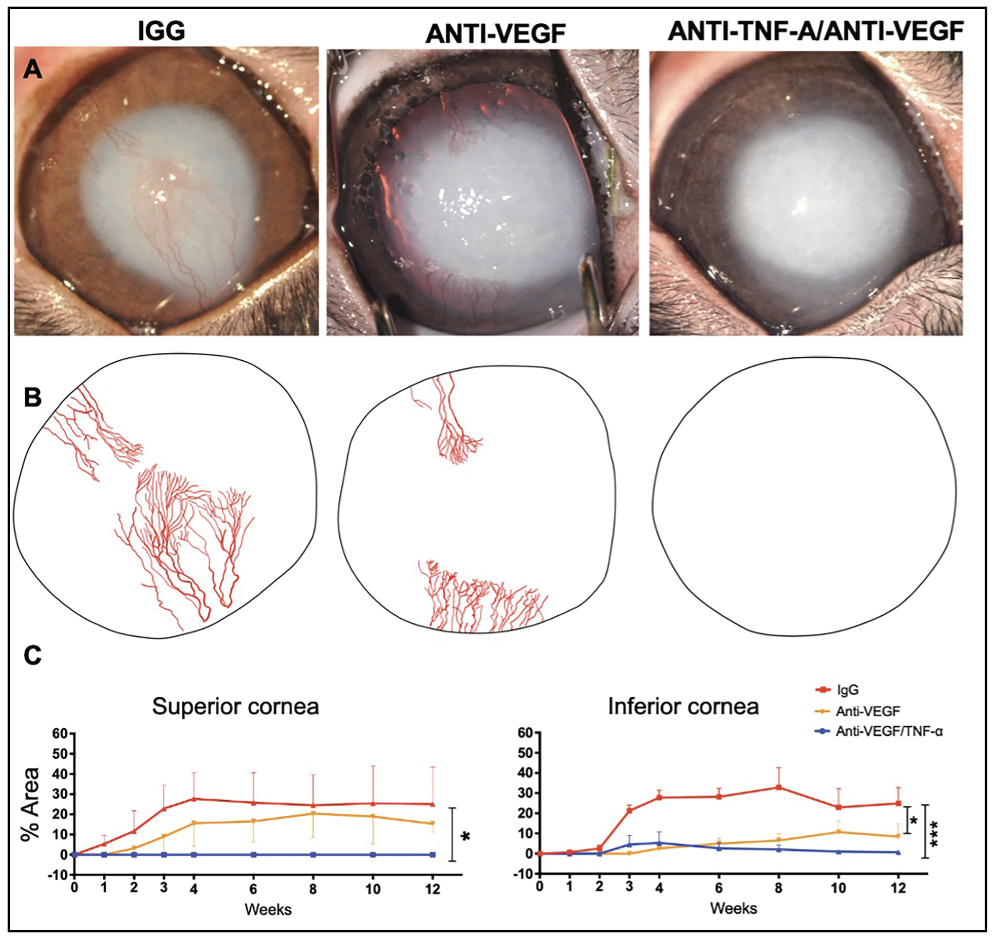 |
| Figure 2. Therapeutic effect of single subconjunctival administration of anti-TNF-a/anti-VEGF antibodies after severe ocular alkali injury. |
Current challenges
Although TNF-α inhibitors are effective against retinal inflammation and degeneration, they’re associated with side effects such as an increased risk of infections. Other complications include the development of anti-drug antibodies, malignancies, potential worsening of uveitis, and high costs. Physicians must weigh these risks against the potential benefits when considering TNF-α inhibitors as a treatment option.
Ongoing research suggests that many of these complications can be mitigated by local administration of the antibody, which may reduce the formation of anti-drug antibodies and lower the cost of the therapy. Additionally, combining TNF-α inhibitors with VEGF inhibitors could further enhance therapeutic potential, as preclinical studies have shown that anti-TNF-α/anti-VEGF therapy is more effective in preventing new vessel growth compared to VEGF inhibitors (aflibercept or bevacizumab), while also providing neuroretinal protection. Using a polymer-based drug delivery system, a single subconjunctival administration of anti-TNF-α (0.7 mg) and anti-VEGF (1.3 mg) offers long-lasting control of vascular growth for over three months.10
Bottom line
TNF-α inhibitors offer promising potential in treating retinal diseases, particularly in preventing retinal degeneration and enhancing the efficacy of VEGF inhibitors against neovascularization. These inhibitors significantly protect the retina and optic nerve from inflammatory damage. Future developments may include novel bispecific antibodies or small molecules targeting both signaling pathways, which could improve the clinical utility of this combination therapy. Additionally, incorporating innovative drug delivery systems could enhance therapeutic outcomes. Other prospects include the development of non-viral gene therapies targeting TNF-α and VEGF, with controllable gene expression for ophthalmic use.
REFERENCES
1. Kropp, Martina, et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—risks and mitigation. Epma Journal. 2023;14:1:21-42.
2. Chaudhuri M, Hassan Y, Vemana PP, Pattanashetty MS, Abdin ZU, Siddiqui HF. Age-related macular degeneration: An exponentially emerging imminent threat of visual impairment and irreversible blindness. Cureus. 2023;15:5:e39624.
3. Romano F, Lamanna F, Gabrielle PH, et al. Update on retinal vein occlusion. Asia-Pacific Journal of Ophthalmology. 2023;12:2:196-210.
4. Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology. 2005;115:1:1-20.
5. Rübsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19:4:942.
6. Aveleira CA, et al. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:11:2872-82.
7. Costa GN, et al. Contribution of TNF receptor 1 to retinal neural cell death induced by elevated glucose. Mol Cell Neurosci. 2012:50:1:113-23.
8. Zhou C, Lei F, Sharma J, et al. Sustained inhibition of VEGF and TNF-α achieves multi-ocular protection and prevents formation of blood vessels after severe ocular trauma. Pharmaceutics. 2023;15:8:2059.
9. Zhou C, Robert MC, Kapoulea V, et al. Sustained subconjunctival delivery of infliximab protects the cornea and retina following alkali burn to the eye. Invest Ophthalmol Vis Sci. 2017;58:1:96-105.
10. Paschalis EI, Zhou C, Sharma J, et al. The effect of subconjunctival TNF-α inhibitors on retinal ganglion cell apoptosis and optic nerve degeneration after corneal surgery or trauma-of possible prophylactic value against secondary glaucoma. Acta Ophthalmol. Sept. 2023;102:3:e381-e394.



