Take-home points
|
 |
Bios Dr. Momenaei is a research fellow at the retina Dr. Hsu is Retina Specialist’s chief medical editor and an associate professor at the Disclosures: Dr. Momenaei has no pertinent financial interests. Dr. Hsu is a consultant to Astellas/Iveric Bio, Gyroscope Therapeutics and receives grant support from Genentech/Roche, Astellas/Iveric Bio, Stealth |
In the spring, just over 11,600 physicians, scientists and members of industry descended on the Emerald City of Seattle to discuss the latest findings in all facets of ophthalmology at the annual meeting of the Association for Research in Vision and Ophthalmology. A good bit of this research was centered on the retina and vitreous.
Here, we’ll take a look at interesting research that ranges from management of post-injection endophthalmitis to novel insights on age-related macular degeneration and geographic atrophy. To catch up on the latest data, read on.
Vitrectomy for post-injection endophthalmitis shows no benefit vs. antibiotics alone
Endophthalmitis has been well-documented as a rare but potentially vision-threatening complication of anti-VEGF injections. Evidence on the best treatment for post-injection endophthalmitis—whether intravitreal antibiotics alone or intravitreal antibiotics along with pars plana vitrectomy—is limited.
A multi-institutional group of investigators in New England performed a retrospective cohort analysis of 1,044 postinjection endophthalmitis patients from 2016 to 2020 entered into the American Academy of Ophthalmology’s IRIS (Intelligent Research in Sight) Registry, with 935 patients in the injection-only group and 109 who had PPV plus antibiotic injections within two days of diagnosis. The study excluded patients who had cataract surgery during the study period, received intravitreal steroids within 30 days of their endophthalmitis diagnosis, or had intermediate/posterior uveitis or cystoid macular edema.
The researchers matched 218 patients—109 from each group—with similar visual acuity at baseline and at the time of endophthalmitis diagnosis. The median logMAR VAs among all matched patients were 0.32 at baseline, 0.88 at diagnosis, and 0.57 post-treatment, Sophia Ghauri, a postgraduate researcher in the lab of Magdalena Krzystolik, MD, at Massachusetts Eye and Ear Infirmary in Boston, reported. Dr. Krzystolik is senior study author.
 |
| For endophthalmitis following an anti-VEGF injection, researchers have found that antibiotics alone are probably the most useful vs. adding an adjunct vitrectomy. |
Post-treatment VA was significantly associated with VA before endophthalmitis (p<0.001) and VA at the time of endophthalmitis diagnosis (p<0.001). No statistically significant differences in the visual outcomes were found between the two matched treatment groups.
Ms. Ghauri is an owner of Codified Health. Dr. Krzystolik has no relevant disclosures.
Ghauri S, Ross C, Ullanat V, et al. ARVO Abstract No. 3826. Early vitrectomy for postinjection endophthalmitis: An IRIS Registry analysis. Invest Ophthalmol Vis Sci. 2024;65:3826.
Mediterranean diet seems to have no impact on GA progression
Researchers at the National Eye Institute analyzed color fundus photographs from annual study visits of participants with noncentral GA in the Age-Related Eye Disease Study to see if dietary intake had any influence on GA progression toward the central fovea. They detected a signal that intake of a number of nutrients, including lutein/zeaxanthin, seems to slow GA progression toward the central macula, but the Mediterranean diet not so much.
The study population consisted of 413 eyes of 347 patients with noncentral GA (88 prevalent, 325 incidental). They analyzed intake of nine different nutrients along with Mediterranean diet index (aMedi), using food frequency questionnaires. Elvira Agron, MS, a statistician at NEI, reported that the following nutrients were associated with slower GA progression toward the foveal center, based on eyes of participants with the highest intake compared to those with the lowest intake:
 |
| NEI research has found that the Mediterranean diet may not have an effect on the progression of geographic atrophy. |
• Zinc, 34.8 (95% CI 29.6,40.1) vs. 45.5 (95% CI 39.9,51.1, p=0.006).
• β-carotene, 29.8 (95% CI 24.2,35.3) vs. 46.6 (95% CI 41.3,51.9, p<0.0001).
• Lutein/zeaxanthin, 32.9 (95% CI 26.2,39.6) vs. 47.5 (95% CI 42.4,52.7, p=0.001).
• Eicosapentaenoic acid (EPA), 35.5 (95% CI 29.2,41.8) vs. 43.7 (95% CI 38.8, 48.6, p=0.05).
The results suggest these nutrients may contribute to the natural phenomenon of relative foveal sparing in GA, which higher dietary intake may augment. Patients with noncentral GA may benefit from dietary advice that targets these specific nutrients, above and beyond general advice on the adoption of a healthy diet, Ms. Agron stated.
Ms. Agron and co-authors have no relevant disclosures.
Agron E, Keenan TDL, Vitale S, Chew EY. ARVO abstract No. 4363. Associations between dietary nutrient intake and geographic atrophy progression towards the central macula. Invest Ophthalmol Vis Sci. 2024;65:4363.
Aflibercept in a drop shows signal in mouse eyes
In preclinical studies, an eye drop has been shown to deliver potentially therapeutic levels of aflibercept in mouse eyes with laser-induced choroidal neovascularization. Venice Chiueh, PhD, director of research development at Wincal Biopharm, the San Francisco-based company investigating the drug-delivery platform, reported on ocular penetrating carrier (OPC) eye drops.
Mice received eye drops for seven days immediately after CNV was induced, or five to seven days post-laser when lesions were analyzed point-to-point over time. The investigators used fundus fluorescein angiography to measure vascular leakage, optical coherence tomography to evaluate lesion volume, and isolectin-B4 staining and imaging to evaluate CNV area. They compared the change in CNV leakage, volume and area of aflibercept-OPC treatment to buffer control, and also compared efficacy of the eye drop to intravitreal injection of aflibercept.
Several OPCs demonstrated what the investigators described as relatively high intraocular penetration of aflibercept in mouse and pig eyes. In mice with laser-induced CNV, the aflibercept drop showed a decrease in leakage, lesion volume and area with efficacy similar to intravitreal injection: a 24.9-percent reduction of leakiness with intravitreal injection and 20.1-percent reduction with OPC compared to control. The company is also investigating topical formulations of brolucizumab, ranibizumab, bevacizumab and faricimab.
Dr. Chiueh and co-authors are employees of Wincal Biopharm.
Chiueh V, Lee L, Tau S, Luu M, Yoo J-S, Lee TW. ARVO Abstract No. 2159. Development of eyedrops for anti-VEGF therapeutic antibodies as a substitute for IVT injection for posterior ocular disease indications. Invest Ophthalmol Vis Sci. 2024;65:2159.
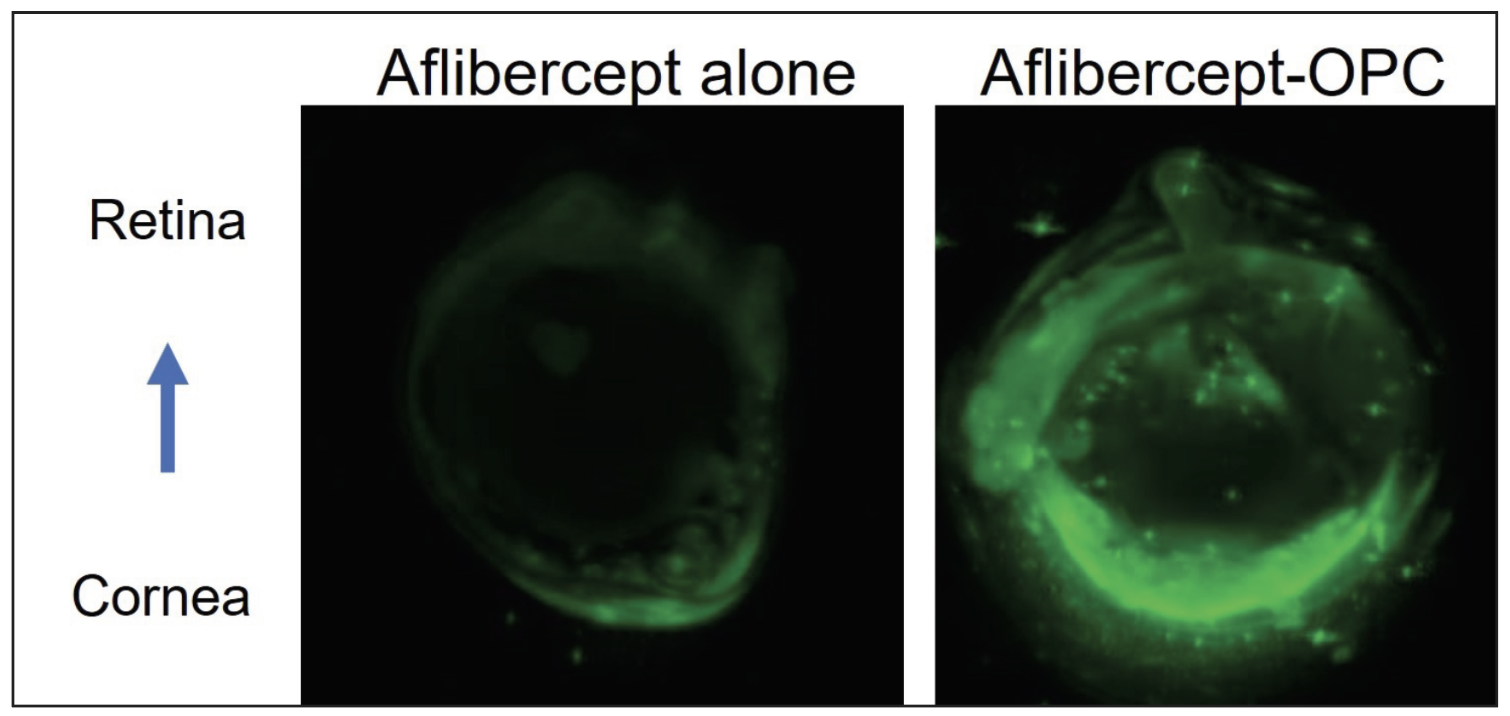 |
| Researchers instilled an experimental aflibercept drop in mouse eyes to analyze the drug uptake. Shown here is a mouse eye section after aflibercept alone or aflibercept-OPC eyedrop. IR800-aflibercept in green. (Chiueh V, Lee L, Tau S, et al. ARVO 2024. Abstract No. 2159.) |
A potential new laser treatment for neovascular age-related macular degeneration
Investigators in Japan reported on their experience using a common cancer treatment, photoimmunotherapy (PIT), to treat age-related macular degeneration. PIT attaches photosensitive antibodies to cells that express specific antigens. Exposure to near-infrared (NIR) light activates the antibodies, inducing targeted cell death while preserving healthy tissue.
The study involved mice with laser-induced CNV. Six days following laser irradiation, they were given Alexa 488-conjugated anti-VEGFR2 antibody systemically, with localization of the antibody confirmed 24 hours after administration.
The next step occurred six days after CNV induction, the antibody-IR700 complex, in which the anti-VEGFR2 antibody was conjugated to the photosensitive IR700 substance, was administered systemically. NIR-PIT was performed by irradiating the retina with NIR light 24 hours after the antibody was administered. Three days after the NIR-PIT, isolectin IB4 staining on the retinal front was done to measure CNV volume.
After 24 hours following administration, the researchers confirmed the presence of the antibody-Alexa 488 complex within the area of CNV. The finding suggests the antibody-photosensitizer complex was localized at the core of the CNV, Hideto Osada, MD, of Keio University in Tokyo, reported at ARVO.
Dr. Osada disclosed a financial relationship with Tsubota Laboratory, as did co-authors.
Osada H, Chen SSW, Guzman N, et al. ARVO abstract 2160. New laser treatment for neovascular AMD targeting VEGFR2 with near-infrared photoimmunotherapy. Invest Ophthalmol Vis Sci. 2024;65:2160.
Optogenetic therapy for retinitis pigmentosa yields BCVA improvement at one year
The Phase IIb/III RESTORE trial is evaluating MCO-010 (sonpiretigene isteparvovec, Nanoscope Therapeutics) gene therapy in patients with advanced retinitis pigmentosa. Allen Ho, MD, director of retina research at Wills Eye Hospital in Philadelphia, reported on 52-week longitudinal data that showed that patients treated with MCO-010 demonstrated improvement in BCVA compared to patients in the sham group.
MCO-010 is a gene mutation-agnostic optogenetic therapy, a multi-characteristic opsin transgene delivered by adeno-associated virus (AAV) 2 through an intravitreal injection. It transduces bipolar cells to express a photosensitive opsin protein that’s designed to restore photosensitivity in people with permanent photoreceptor loss.
Study participants had advanced RP with baseline VA <1.9 logMAR (Snellen equivalent: 20/2000) in the study eye and ≤1.6 logMAR (Snellen equivalent: 20/800) in the fellow eye. Participants were grouped into three treatment arms: a low-dose arm that received a single dose of 0.911 gc/eye (n=9); a high-dose arm that received a single dose of 1.211 gc/eye (n=9); or sham (n=9). VA was evaluated systematically until week 52 using Freiburg VA.
Both treatment arms demonstrated greater improvement compared to sham in BCVA at weeks 16, 24, 36 and 52. In the low-dose arms, mean improvement of BCVA at the respective intervals were 0.171, 0.207, 0.438 and 0.337 logMAR (p=0.2242, 0.1424, 0.0021 and 0.0164) compared to sham. In the high-dose arm, mean improvement of BCVA was 0.077, 0.220, 0.228 and 0.301 logMAR (p=0.6016, 0.1277, 0.1091 and 0.0355) compared to sham.
Dr. Ho also reported that MCO-010 was also well-tolerated with no serious adverse events observed through week 52.
Dr. Ho disclosed he is a consultant and contractor for Nanoscope Therapeutics.
Ho A. Longitudinal BCVA analysis of low- or high-dose MCO-010 mutation agnostic optogenetic therapy for retinitis pigmentosa: 12-month results from a Phase 2b/3 randomized, sham-controlled, patient- and assessor-masked clinical trial (RESTORE). Invest Ophthalmol Vis Sci. 2024;65:2137.
An updated AREDS scale for late age-related macular degeneration
The AREDS Simplified Severity Scale (SSS) for five-year risk of progression to late AMD has undergone the following three updates, Tiarnan D.L. Keenan, MD, PhD, of the National Eye Institute, reported: the definition of late AMD to include noncentral GA; incorporating reticular pseudodrusen (RPD); and performing an external validation of AREDS2.
The AREDS AMD SSS underwent two significant updates. For individuals without RPD, the new 0–4 scale for five-year progression rates is similar to the original scale: around 0.5, 4, 12, around 25 and 50 percent. For those with RPD, the new scale for five-year progression is approximately double for most level: 3; 8; around 30; around 60 and around 70 percent.
Dr. Keenan reported that the new scale fits modern definitions of late AMD, has increased prognostic accuracy and appears generalizable between study applications, but remains simple enough for broad risk categorization.
The AREDS researchers arrived at the new scale by evaluating five-year progression rates to late AMD among AREDS (n=2,719) and AREDS2 (n=1,658) study participants with no late AMD at baseline. They calculated five-year progression rates to late AMD according to levels 0–4 on the SSS after two updates: noncentral GA was now considered part of the outcome rather than a risk feature; and the scale was divided by RPD status at baseline.
In AREDS, following the first scale update, the five-year rates of progression to late AMD for levels 0–4, respectively were 0.3, 4.5, 12.9, 32.3 and 55.6 percent. Following both updates, the proportion progressing to late AMD at five years was 8.4 percent without RPD and 40.6 percent with RPD.
As the final SSS, the five-year progression rates for levels 0–4, respectively, were 0.3, 4.3, 11.6, 26.7 and 50 percent without RPD, and 2.8, 8, 29, 58.7 and 72.2 percent with RPD. In external validation on AREDS2, for levels 2–4, the progression rates were similar, at 15.3, 30.4 and 45.7 percent (without RPD) and 27.3, 47.9 and 73 percent (with RPD), respectively
The study authors have no relevant disclosures.
Keenan TDL, Agron E, Domalpally A, Cukras CA, Chew EY. The Updated AREDS simplified severity scale for age-related macular degeneration, incorporating reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2024;65:2790.
Early test of suprachoroidal delivery of an AAV vector to inhibit complement pathway
A number of studies are evaluating the efficacy of a range of ocular drugs administered into the suprachoroidal space, but researchers from RegenXbio have reported on the first experience using the suprachoroidal space to deliver gene therapy.
Brendan Lilley, PhD, principal scientist at RegenXbio, reported that the researchers developed multiple formats of complement C5 inhibitors designed for AAV vectors. They evaluated the binding kinetics, affinity and bioactivity toward C5 from human, cynomolgus macaque nonhuman primate (NHP) and mouse.
The researchers transduced Human iPSC-derived retinal pigment epithelium cells with AAV vectors expressing C5 inhibitors and they measured the inhibition of membrane attack complex (MAC) using immunohistochemistry. They examined expression in vivo in mice after subretinal injections.
They also tested a format optimized for ocular biodistribution for expression and tolerability in NHP following with a subretinal injection with a one-month duration or suprachoroidal administration for three months. They used in vivo imaging and processed a subset of eyes for histopathology to evaluate tolerability. In subretinally injected eyes, they examined the bioactivity of in vivo expressed inhibitor in NHP vitreous humor.
Dr. Lilley reported that C5 inhibitors showed a high affinity for human and NHP ligands, and potentially suppressed complement activation. Transduction of human induced pluripotent stem cell-derived RPE cells with vectorized C5 inhibitors reduced MAC formation on the cell surface.
The lead C5 inhibitor demonstrated improved expression and distribution to outer ocular layers following subretinal delivery in mice. In NHPs, the one-month study following subretinal delivery and the three-month study of suprachoroidal delivery both showed the lead AAV vector was well-tolerated and yielded high expression of bioactive C5 inhibitor in ocular fluids and tissues (including the RPE/choroid) with minimal levels of C5 inhibitor in serum.
The study showed that suprachoroidal delivery of an AAV vector encoding a complement inhibitor optimized for ocular expression resulted in localized and durable expression of bioactive protein, Dr. Lilley stated. This represents a potential minimally invasive approach to treat dry AMD to reduce treatment burden and deliver therapeutic molecules directly to the site of AMD pathogenesis. RS
Dr. Lilley and all co-authors are employees of RegenXbio.
Lilley B, Lee WH, Giles A, et a. Delivery to the suprachoroidal space of an AAV vector encoding a complement pathway inhibitor as a possible treatment for dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2024;65:1941.



