Take-home points
|
 |
Bios Dr. Mishev is with Focus Eye Centre Sofia in Sofia, Bulgaria. Dr. Abreu-Arbaje and Dr. Sosa-Lockward are with Hospital Dr. Elías Santana, Santo Domingo, Dominican Republic. Dr. Gibson is with the department of ophthalmology at Emory University, Atlanta. Dr. Nguyen is with the University of Alabama Birmingham Heersink School of Medicine. Dr. Franklin is with the Diagnostic and Medical Clinic in DISCLOSURES: Dr. Mishev disclosed a financial relationship with Alcon. Dr. Abreu-Arbaje reported financial relationships with Alcon, Bayer, Ocutrx and Roche/Genentech. Dr. Lockwood, Dr. Gibson, |
Intraoperative fluorescein angiography can help to improve visualization of biomarkers during vitrectomy surgery, but its use seems to be more aspirational than practical. The advent of three-dimensional, high-definition surgical visualization promised to take IOFA during digitally assisted vitreoretinal surgery (DAVS) for pars plana vitrectomy for proliferative diabetic retinopathy and retinal vein occlusion in a new direction.6,7 Here, we report on our modified approach that uses an optical exciter filter and either an optical or digital barrier filter to make it easier to switch to IOFA during vitrectomy surgery.8
Light sources and optical filters
The optical exciter filter can be placed in a number of different light sources, and a variety of filters are available.9-11 To achieve the optimal fluorescein excitation, an optical filter with a dedicated wavelength of 475 to 490 nm should be inserted into the illumination light source. Different light sources have different spectrograms (Figure 1).
Based on the different emissions, we’ve found the Constellation Xenon (Alcon) illumination source has the best characteristic to detect fluorescein emissions. Spectrograms (Figure 2) demonstrate that Clarity 475 and Semrock 475 optical filters produce the best signal for fluorescein excitation. More recently, an LED light source from Geuder was shown to produce quality visualization as well.
Enhanced contrast is the key element of successful IOFA (Figure 3). To achieve this, we determine the optimal excitation source to maximum fluorescein signal intensity.
 |
| Caption |
To create the optimal digital barrier filter recipe with an additional Notch laser 532 filter, a high dynamic range and sensitivity camera is essential to create an optimal digital barrier filter combination because the 532 laser filter can reduce the fluorescence intensity as it coincides with the wavelength emission of the fluorescein, 510 to 540 nm.
Based on our experience, we’ve found that the Purepoint 532 (Alcon) laser filter may suppress fluorescence emission less than the Iridex 532 filter—based on spectrograms.12 The Purepoint 532 filter also has swivel in-and-out design, so it can be removed during IOFA and not block any fluorescein emission. When we need to perform IOFA-guided endolaser, we engage the Purepoint filter and increase the gain on the camera to improve the image quality.
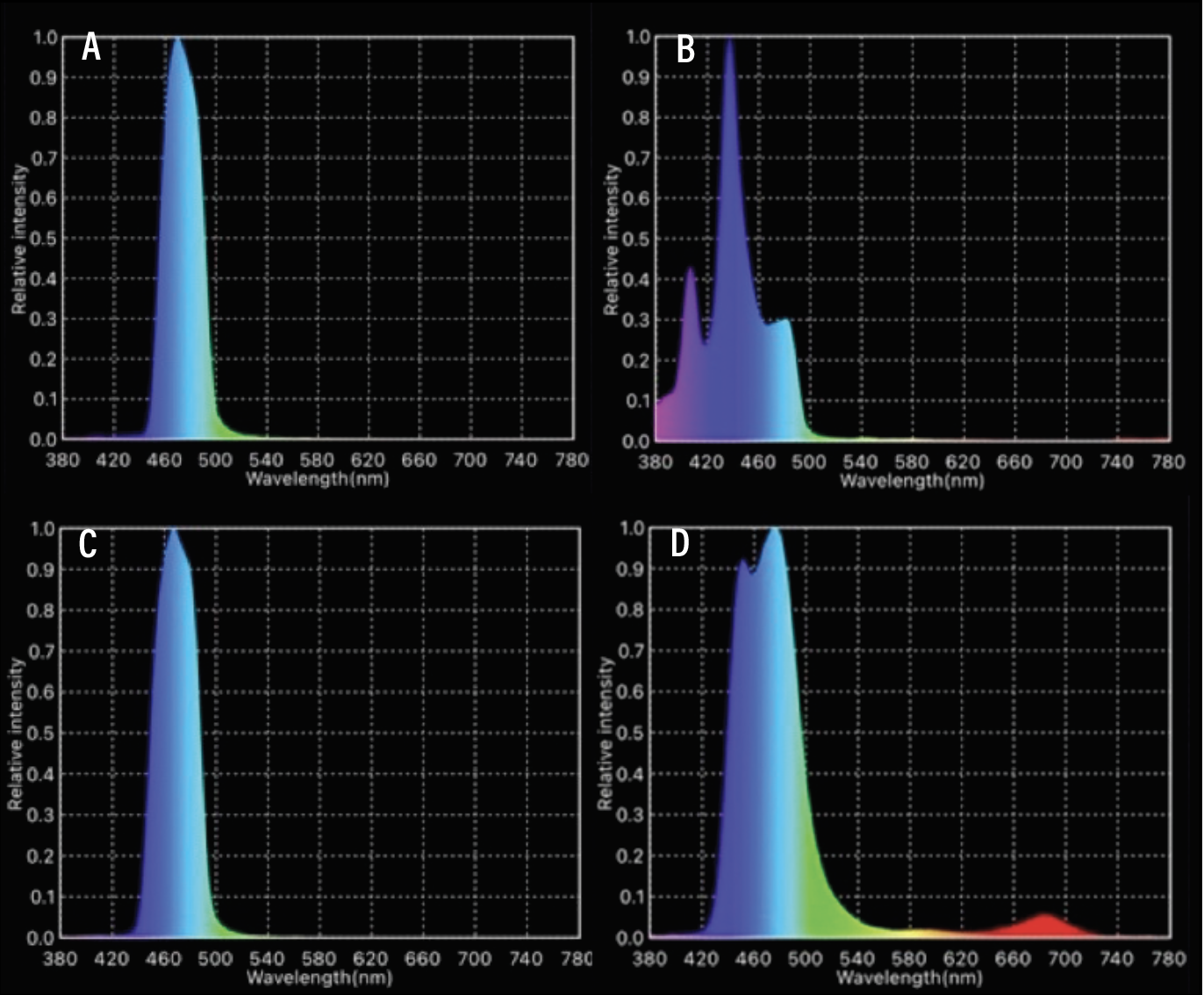 |
| Figure 2. Spectrograms achieved by four different excitation filters ranging from 475 to 490 nm: A) Clarity 475; B) Omega 490; C) Semrock 475; and D) Leica 476. We chose to work with Clarity 475 and Semrock 475, due to their optimal peak wavelength and bandwidth. Omega 490 showed a spectrogram with peak on 440 nm and low emission in the desired 475-to-490 nm wavelength. Leica 476 emits more broadband in full color spectrum, which can potentially reduce the overall image contrast. |
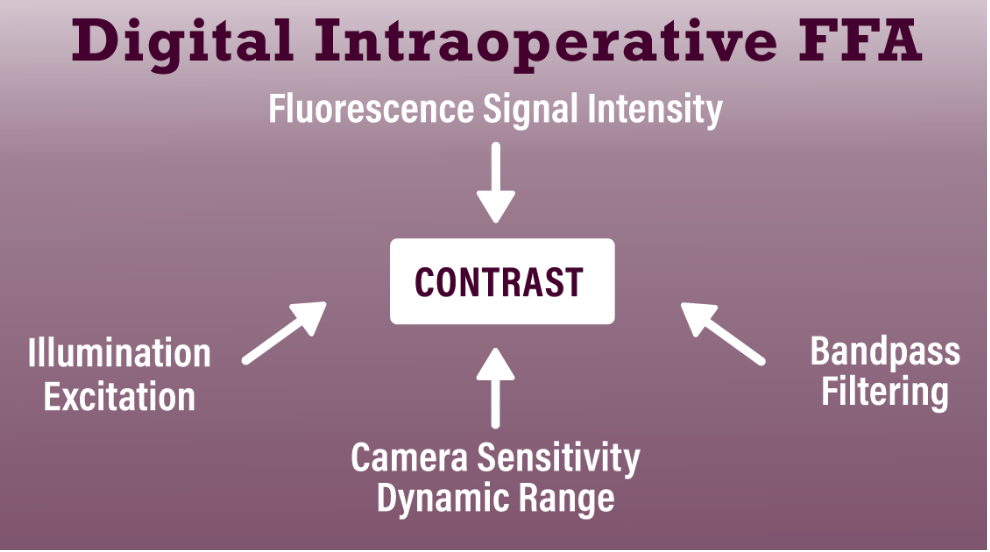 |
| Figure 3. Schematic showing the components of intraoperative fluorescein angiography that modify contrast, which is critical for optimal visualization. |
Digital barrier filters and signal
 |
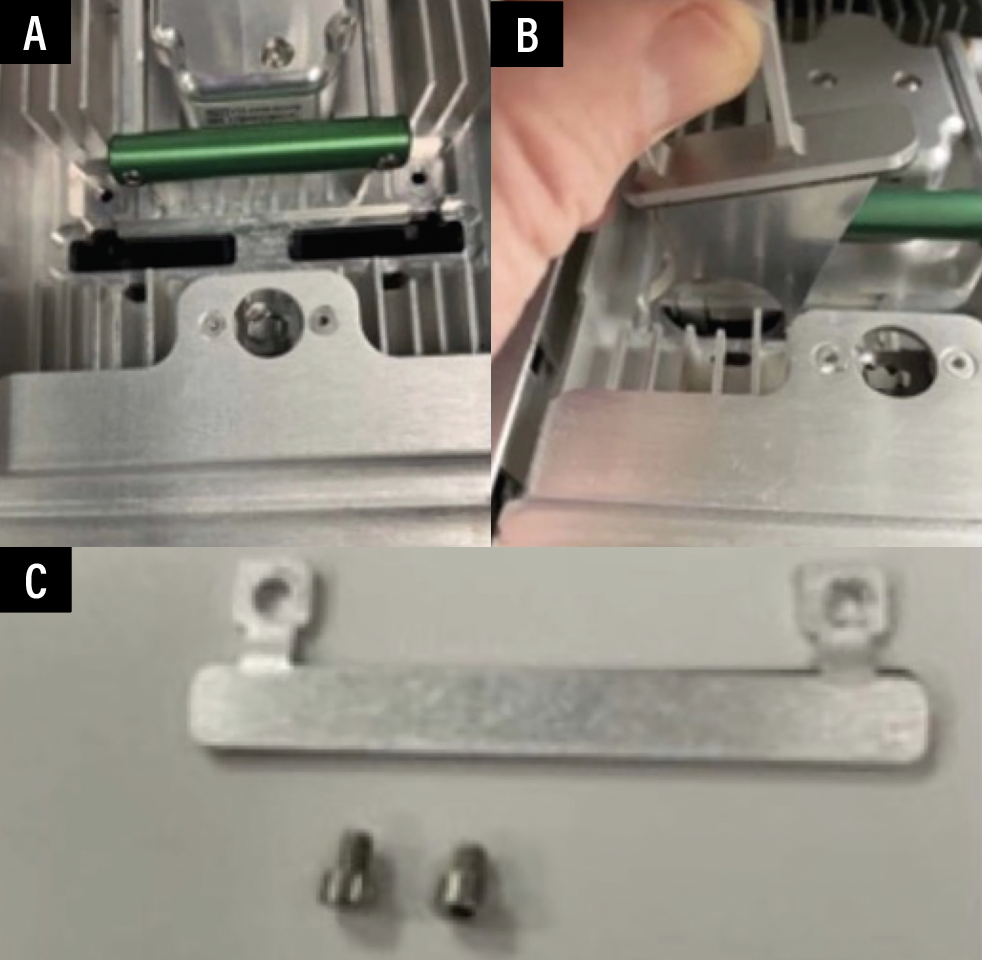
| Figure 4. Detail of the optical barrier filter placed into the washer. |
The digital barrier filter works by reducing the red and blue emissions to enhance the green signal. Then it alters the saturation and hue to diminish the blue-green color, enhancing the signal with brightness and contrast to produce a grayscale image similar to that from office-based fluorescein angiography (Figure 4).
The Ngenuity Version 1.5 platform (Alcon) can permit the operator to use computer modeling to optimize the signal once the operation is finished. Thus, the signal will be enhanced further secondary to the ongoing modifications of both the optical exciter and digital barrier output in this and other emerging DAVS platforms.
Both optical and digital barrier filters produce an excellent signal (Figure 5). So, if you don’t have access to digital visualization, an optical barrier filter will work well with a standard optical microscope.
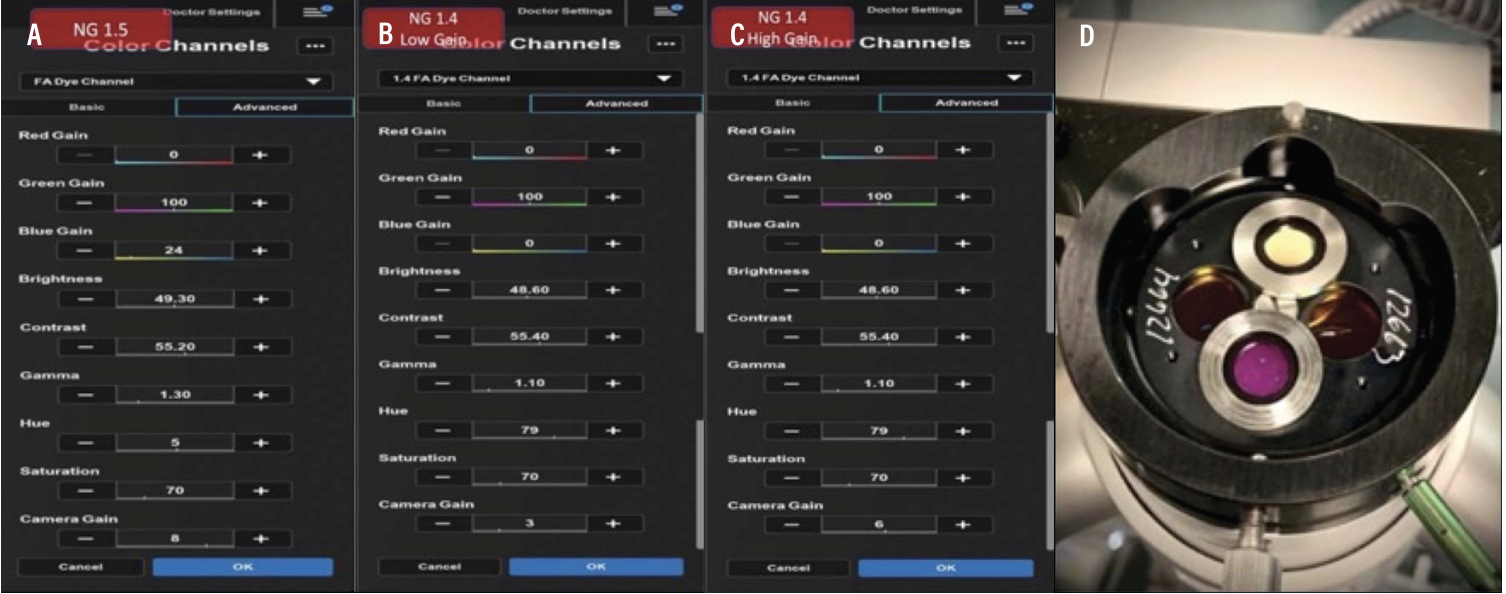 |
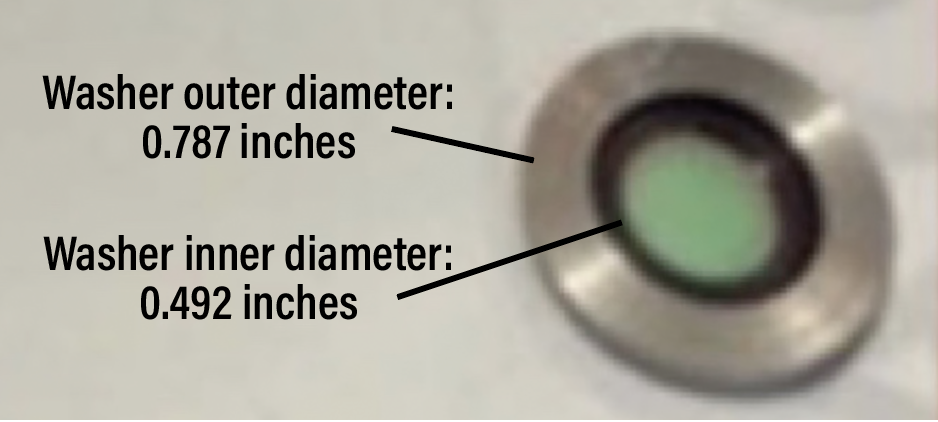
| Figure 5. Optical barrier filter placement and digital barrier filter specifications. A, B, C) Specific color channels developed for intraoperative fluorescein angiography using Ngenuity for both software versions 1.4 and 1.5 showing the specifications of the digital filters or color channels. D) Placement of the optical filter and washer into the switchable laser filter. |
Machine and microscope setup
Although, the current IOFA protocol requires an initial setup, once the setup is complete, subsequent IOFA can be done rather seamlessly. Both the optical microscope and digital camera parameters must be optimized before adopting IOFA. This involves a simple task at the beginning of each day: properly cleaning and focusing the optical microscope and white light exposure or balance of the digital camera.
Once this is done, the switch to IOFA takes about 30 seconds and only involves switching the light to the source to where the optical exciter filters are located and changing the digital surgical channel or optical filter in the microscope. The switch back to standard visualization once IOFA is complete is equally efficient.13 We’ve found that dual light output with a chandelier light source and a light pipe endoilluminator produces the best signal.
Surgical decision-making
In our experience IOFA provides additional feedback during vitrectomy surgery that can guide and enhance decision-making.14 For example, it can detect a delay in vascular filling time when a patient’s blood pressure drops or intraocular pressure spikes, or if both happen.13
IOFA clearly visualizes vascular epiretinal membranes, maximizing the contrast between the vascularized fluorescent vessels against a dark background, which can be helpful to identify the correct surgical plane to delaminate. And it clearly visualizes both areas of residual abnormal vascularities and retinal ischemia when the delamination is completed.
To reduce the risk of postoperative vitreous hemorrhage while maximizing peripheral and night vision,15 we treat these residual areas of abnormal vascular leakage with confluent laser, and place more confluent laser in the areas of peripheral ischemia while sparing the better perfused retina. IOFA can also be helpful in diagnosis and management, and ultimately guide surgical decision-making, when patients have media opacities, such as blood or inflammatory cells that occlude visualization of fluorescein biomarkers during the preoperative evaluation.
Safety and phototoxicity
The safety of FA has been well documented. The cutoff is 440 nm wavelength: toxicity could result below that. The bandpass exciter filters have a wavelength of 470 to 485 nm.16,17
However, the straight light pipe has been reported to cause light toxicity if it’s held close to the retina for more than 15 minutes.18 Because the fluorescein signal diminishes significantly after five to 10 minutes, the risk of significant phototoxicity is low.
Some authors have suggested that fluorescein may enhance laser burn intensity, but no reports exist of excessive laser burning with macular laser after FA in the clinic. Photocoagulation burns are rarely placed near the macular center.
In any event, we’ve found that a chandelier light source plus a light pipe endoilluminator held away from the retinal surface reduces the risk of any phototoxicity.19 The International Commission on Non-Ionizing Radiation Protection (ICNIRP) published the “blue light hazard function,” a wavelength-dependent weighting function, and guideline exposure limits.20
In more than 200 cases with IOFA, no light toxicity has been reported. However, it’s important to proceed with clinical studies that confirm the lack of toxicity by measuring multiple different safety parameters postoperatively. They include vision, spectral-domain optical coherence tomography, multifunctional electroretinogram and visual fields to provide objective data on phototoxicity.
The future of IOFA
IOFA better delineates vascular preretinal membranes, and helps surgeons modify their intraoperative laser treatment.
Early data from 88 eyes that had IOFA-guided surgery demonstrate both improved vision and reduction in the rate of postoperative vitreous hemorrhage compared to standard vitrectomy: 10 to 12 percent vs. 30 to 40 percent. This helps to validate the potential benefits of adding IOFA to the surgical armamentarium during vitrectomy surgery.
Future efforts will aim to document safety measures, such as contrast sensitivity, multifocal ERG, microperimetry and visual fields in eyes that undergo IOFA. In addition, potential benefits may be associated with IOFA-guided laser for retinal vascular disease compared to standard panretinal laser.
Further, advances with optical exciter and digital barrier filters should lead to improved and more useful information. Thus, we believe that IOFA is an important developing technology to assist decision-making during vitrectomy surgery and ultimately will enhance patient outcomes.
Acknowledgments: The authors acknowledge the work of Jared Ridgeway, Miachanel Nguyen, Mariam Omar, Hudson Tate, Brenton Bickell and Ariel Shin for data entry and analysis.
REFERENCES
1. Charles S. Vitreous Microsurgery. Baltimore: Williams & Wilkins, 1981.
2. Avery RL, Hickingbotham D, Jaffe G, de Juan E Jr. Intraoperative fluorescein. Arch Ophthalmol. 1992;110:11:1518-1519.
3. Googe JM, Bessler M, Hoskins JC, Miller JH. Intraoperative fluorescein angiography. Ophthalmology. 1993;100:1167-1170.
4. Krueger RR, Morales RB, Smith RE et al. New stroboscopic light source for intraoperative retinal fluorescein angiography. Arch Ophthalmol. 1994;112:420-422.
5. Terasaki H, Miyake Y, Awaya S. Fluorescein angiography of peripheral retina and pars plana during vitrectomy for proliferative diabetic retinopathy. Am J Ophthalmol. 1997;123:370-376.
6. Imai H, Tetsumoto A, Inoue S et al. Intraoperative three-dimensional fluorescein angiography-guided pars plana vitrectomy for the treatment of proliferative diabetic retinopathy. Retina. 2023;43:359-362.
7. Imai H, Matsumoto A, Yamada A et al. Intraoperative three-dimensional fluorescein angiography-guided pars plana vitrectomy for branch retinal vein occlusion. Retina Cases Brief Rep. 2022;16:802-805.
8. Mishev L. Real time 3D intraoperative fluorescein angioscopy with Alcon Ngenuity custom digital filtering [Video]. YouTube. https://youtu.be/4-K-PlBiR6w?si=0-Lt-fQTwMcAZclF. Published August 24, 2022. Accessed June 19, 2024.
9. Delori F, Ben-Sira I, Trempe C. Fluorescein angiography with an optimized filter combination. Am J Ophthalmol. 1976;82:559-66.
10. Hyvärinen L, Hochheimer BF. Filter systems in fluorescein angiography. Acta Ophthalmologica Scandinavica. 1996;74:364-368.
11. Grace J, Edlou S, Foss J, Hodgson C, Rheault JP, Rosvold J, Sieber K, Walters S. Using optical interference filters to measure autofluorescence in substrates and coatings. Surface and Coatings Technology. 2021;426:127777.
12. McCannel CA. Illumination considerations for vitreous surgery. In: Saxena S, Meyer CH, Ohji M, Akduma L, eds. Vitreoretinal Surgery. London: Jaypee Brothers, 2012.
13. She H-C, Zhang X-F, Zhang Y-P, Jiao X, Zhou H-Y. Peripheral arterial filling time and peripheral retina fluorescence features in ultra-widefield angiography. Int J Ophthalmol. 2021;14:1034-1040.
14. Littlewood R, Mollan SP, Pepper IM, Hickman SJ. The utility of fundus fluorescein angiography in neuro-ophthalmology. Neuro-Ophthalmology. 2019;43:217-234.
15. Tan S, Orabona G, Robins J, et al. "Delamination Plus": A technique to reduce immediate postoperative diabetic cavity hemorrhage. Retina. 2023;43:520-522.
16. Youssef P, Sheibani N, Albert D. Retinal light toxicity. Eye. 2011;25:14.
17. van Beurden F, van Willigen DM, Vojnovic B, et al. Multi-wavelength fluorescence in image-guided surgery, clinical feasibility and future perspectives. Mol Imaging. 2020;19:1536012120962333.
18. Van Den Biesen P, Berenschot T, Verdaasdonk R, et al. Endoillumination during vitrectomy and phototoxicity thresholds. Br J Ophthalmol. 2000;84:1372-5.
19. Meershoek P, KleinJan GH, van Willigen DM, et al. Multi-wavelength fluorescence imaging with a da Vinci Firefly—a technical look behind the scenes. J Robotic Surg. 2021;751–760.
20. International Commission on Illumination. Position statement on the Blue Light Hazard (April 23, 2019). CIE. Available at: https://cie.co.at/publications/position-statement-blue-light-hazard-april-23-2019. Accessed July 30, 2024.



