Genentech recently announced that it will be reintroducing its sustained-release ranibizumab implant, Susvimo (ranibizumab injection), onto the market for the treatment of wet age-related macular degeneration. This comes following a voluntary recall of the device in October of 2022.
Susvimo is implanted surgically and refilled once every six months with a needle designed specifically for the implant. It was initially approved by the FDA in 2021 for the treatment of wet AMD in eyes with at least two prior anti-vascular endothelial growth factor injections.
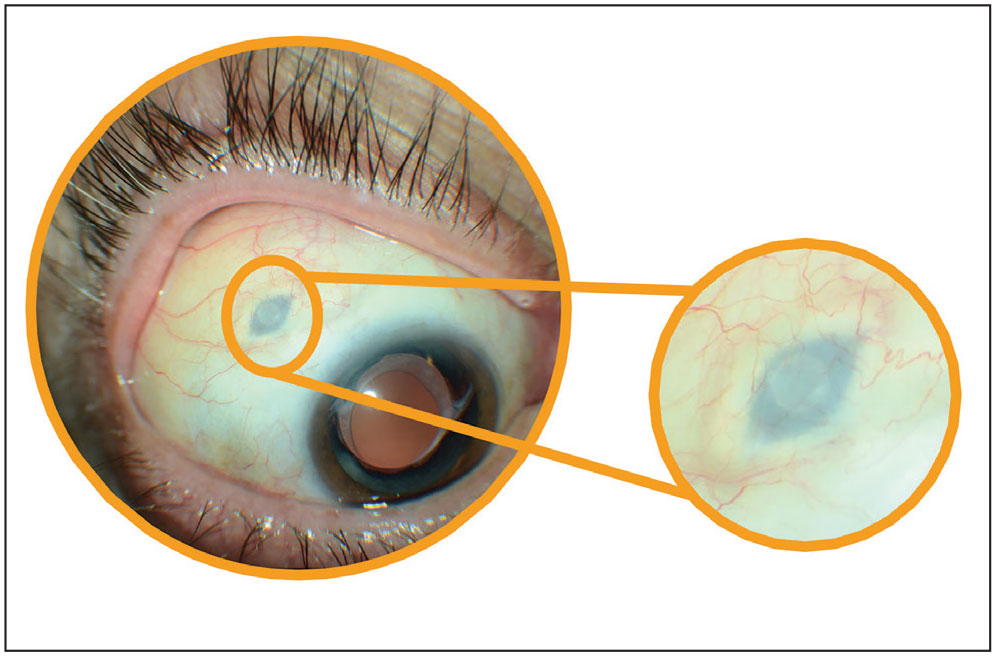
Genentech took the implant and tool assembly kit, including the drug vial and initial fill needle, off the market after it investigated reports of septum dislodgement in the port delivery system in patients during the Phase III trial program. The recall didn’t include the Susvimo 100 mg/ml drug vial or refill needle in order to allow retina specialists to continue refill-exchange procedures in those patients with an existing implant.
 |
In a letter sent to health-care providers first announcing the recall, Genentech stated, “During an investigation into septum dislodgement cases in the port delivery system with ranibizumab Phase III clinical trial program, we identified a need for additional testing of the commercial implant supply. This additional testing of our commercial supply involved repeatedly puncturing Susvimo implants with a needle, to evaluate performance of the septum of the implant over the long-term via multiple refills. The results showed that some implants did not perform to our standards. Hence, a pause in all new implantations is required.”1
According to Jason Hsu, MD, Retina Specialist’s chief medical editor and in practice at Wills Eye Hospital in Philadelphia, who was part of the Phase III trials for Susvimo, as the company alluded to in its statement, the implant includes a septum for the needle to penetrate.
“The septum creates a barrier to prevent any efflux of the drug into the subconjunctival space,” he explains. “However, in the reports of dislodgement, the glue of the septum wasn’t holding, essentially causing the septum to drop into the tip of the implant and prevent any refills.”
Among the changes to the implant were the doubling of the bonding strength of the septum component, as well as lubrication of the refill needle, which allows it to be inserted more smoothly and reduces the insertion force, notes Dr. Hsu.
“In my experience inserting these devices, septum dislodgement wasn’t a major issue,” he says. “We did see it happen in a couple of patients, but we witnessed dislodgement of the entire implant into the eye during the refill procedures. Some of that had to do with the amount of pressure it took to insert the needle into the device for refills. The changes made to the refill needle add a little bit more reassurance that it won’t require as much pressure, so this should hopefully make it much safer for the long term.”
The U.S. Food and Drug Administration has given a post-approval supplement to the Biologics License Application for Susvimo, reflecting these updates. Genentech also said it will work to make Susvimo available in the United States in the coming weeks.
“As for those who did have the original Susvimo implant, now that a new device is Food and Drug Administration approved, they’ll need to have it exchanged at some point, which is a bit of an inconvenience to undergo another surgery,” continues Dr. Hsu. “But I think these long-lasting, anti-VEGF options are a huge benefit to everyone, patients and clinicians, in terms of burden of care. I’m happy to see it’s back on the market because I think it will allow us to have another option for patients who need very frequent treatments. This potentially means they don’t need to come back to the office as often for repeated intravitreal injections.”
REFERENCE
1. Voluntary recall of the SUSVIMO Ocular Implant. https://www.gene.com/download/pdf/Susvimo_DHCP_Important_Prescribing_Information_2022-10-18.pdf. Accessed July 18, 2024.
FDA Approves First OCT Device for At-home Use
Patients with neovascular AMD are often subject to frequent office visits so that eye care providers can monitor anatomical changes and signs of disease progression on OCT—but what if physicians could perform this imaging remotely? This is now possible with the recent FDA approval of the first at-home OCT from Notal Vision, marketed as “Scanly,” which uses artificial intelligence software to evaluate various imaging biomarkers in nAMD. By analyzing scans remotely between scheduled visits, eye doctors may be able to gain additional insight into patients’ disease status, while also cutting back on their travel time.
 |
The company explains in its press release that the device captures spectral-domain OCT images in a 10x10-degree area centered on the point of fixation. Once scans are complete, the AI software is used to segment and estimate the volume of hypo-reflective spaces. All images are automatically transmitted via a built-in wireless connection and stored in the Notal Health Cloud for analysis. There, physicians can review data and set eye-specific notification criteria (including a volume threshold for total retinal hypo-reflective spaces), as well as receive notifications via a web portal.
Two pivotal U.S. trials involving more than 500 patients with nAMD (mean age: 77) were completed to assess the accuracy and user-friendliness of the home OCT device. Notal Vision reports that 97 percent of the total 5,426 scans performed by patients in the study eye were successful. The company further noted an adherence rate of 5.9 scans/week, and on average, patients took 48 seconds to self-image.
To have a Scanly Home OCT device shipped to a patient’s home, the patient must first enroll in what Notal Vision calls the “Scanly Home OCT Monitoring Program.”
For more information on the program or device itself, go to notalvision.com/services/scanly-oct.
Warfarin May Increase Risk of Wet AMD Conversion
A recent study published in Ophthalmology highlighted the delicate balancing act of preventing life-threatening cardiac events and sight-threatening diseases when it reported that warfarin significantly increased the risk of conversion to neovascular AMD.1
A newer class of blood thinners called direct oral anticoagulants (DOACs) have proven superior in several studies compared with traditional warfarin and are preferred first-line agents for stroke prevention in certain cases. However, all anticoagulants are associated with bleeding risks, and DOACs’ intraocular complications aren’t clear yet. Researchers sought to understand the risks in patients with higher bleeding risk such as those with AMD.
The study included patients with non-neovascular AMD initiated on DOACs (n=20,300) or warfarin (n=13,387). Researchers found that at six months and at one year, patients treated with warfarin had a higher risk of developing neovascular AMD compared with those who were treated with DOACs. They also reported that warfarin-treated patients had an increased risk of requiring intravitreal anti-VEGF therapy and pars plana vitrectomy.
Additionally, patients with AMD and atrial fibrillation had an increased risk of ocular complications and need for anti-VEGF therapy over a five-year period. The researchers reported no significant difference in the development of major systemic hemorrhagic events in the two groups over five years.
Based on the real-world study findings, the researchers wrote that patients with non-neovascular AMD on warfarin are at an increased risk for developing ocular complications such as neovascular disease, macular hemorrhage and vitreous hemorrhage, and are more likely to require anti-VEGF therapy or surgical vitrectomy than patients on DOACs.
“Furthermore, there was an observed increased risk of these same events when evaluated over an extended continuous five-year period in a cohort of individuals with dry AMD receiving the anticoagulation for the indication of chronic atrial fibrillation,” the researchers added in their paper.
They concluded that “switching oral anticoagulation from warfarin to select FDA approved DOACs in patients with subsequent neovascular AMD and/or history of neovascular AMD must be considered carefully, given the improved bleeding profile highlighted in the present study.”
REFERENCE
1. Alsoudi AF, Koo E, Wai K, et al. Risk of ocular neovascular conversion and systemic bleeding complications in patients with AMD on DOACs or warfarin. Ophthalmology 2024. [Epub ahead of print].
The Pandemic's Effect on DR Screening
Researchers have found that the number of patients being screened for diabetic retinopathy continues to lag behind pre-pandemic levels.1
Investigators analyzed health claims in central Massachusetts from 2018 to 2022 to compare the number of DR screenings from the years before and after the COVID-19 virus became widespread. The data showed that post-pandemic weekly DR screenings in the region decreased by 15.1 percent compared to pre-pandemic rates. Adjusting for seasonal variation, the study authors reported a post-lockdown screening rate that was 12 percent lower than the mean weekly DR screening rate during the pre-pandemic period. Established patient DR screenings saw a significant decline after the pandemic while no differences were seen for new patients.
The driving forces behind DR screening rates remain unclear, but the researchers wrote in their paper that “it is likely a combination of factors, such as patient behavior and health care resource availability.”
As to why established patients were disproportionately impacted, the researchers wrote in Ophthalmic Epidemiology that “health-care providers may have prioritized new patients for DR screening appointments after the pandemic.” It’s also possible, they add, that established patients with longer duration of diabetes “may have had more advanced diabetes, which is associated with increased comorbid conditions.” RS
REFERENCE
1. Bilsbury E, Wizentier MM, Wood E, et al. The continuing impact of the COVID-19 pandemic on diabetic retinopathy screenings. Ophthalmic Epidemiology 2024. [Epub July 31, 2024]



