Take-home points
|
 |
Bios Ankur M. Shah, MD is a retina specialist at Retina Partners Midwest at Midwest Eye Institute. Raj Maturi, MD has been a clinician and clinical trialist with Retina Partners Midwest for the last 25 years. DISCLOSURES PERTAINING TO THE MATERIAL: DR. Shah is a consultant to Eyepoint. He's done research sponsored by Ocular Therapeutix. Dr. Maturi has done research for Clearside and Ocular |
Treatments for exudative age-related macular degeneration and diabetic retinopathy and diabetic macular edema have advanced tremendously since the era of blasting the macula with thermal laser and full fluence photodynamic therapy. The current generation of anti-vascular endothelial growth factor agents have worked remarkably well in both preserving visual acuity and improving vision in a majority of our patients. These improvements have allowed our patients to continue to read, drive and work. While we retina specialists and our patients have had access to an improving array of anti-VEGF treatment options, vision can quickly be lost if patients don’t adhere to the prescribed long-term treatment plans. Innovative options such as the Port Delivery System for ranibizumab (Genentech) have been helpful for a small subset of patients, but the voluntary recall has kept many patients from receiving this valuable long term therapy.
Recent additions to the marketplace such as high-dose aflibercept (Eylea HD; Regeneron) and faricimab (Vabysmo; Genentech) have offered improved anatomic effects and durability for many patients via higher molar doses and concentrations or dual mechanism inhibition of VEGF and Angiopoietin-2, respectively. While these new options have been excellent additions to our armamentariums, numerous companies and investigators continue to search for even more durable treatments to reduce the burden on our patients, potentially circumventing or at least alleviating, the challenges of patient noncompliance and treatment fatigue. This article will discuss the promising class of molecules known as tyrosine kinase inhibitors.
TKIs in Development
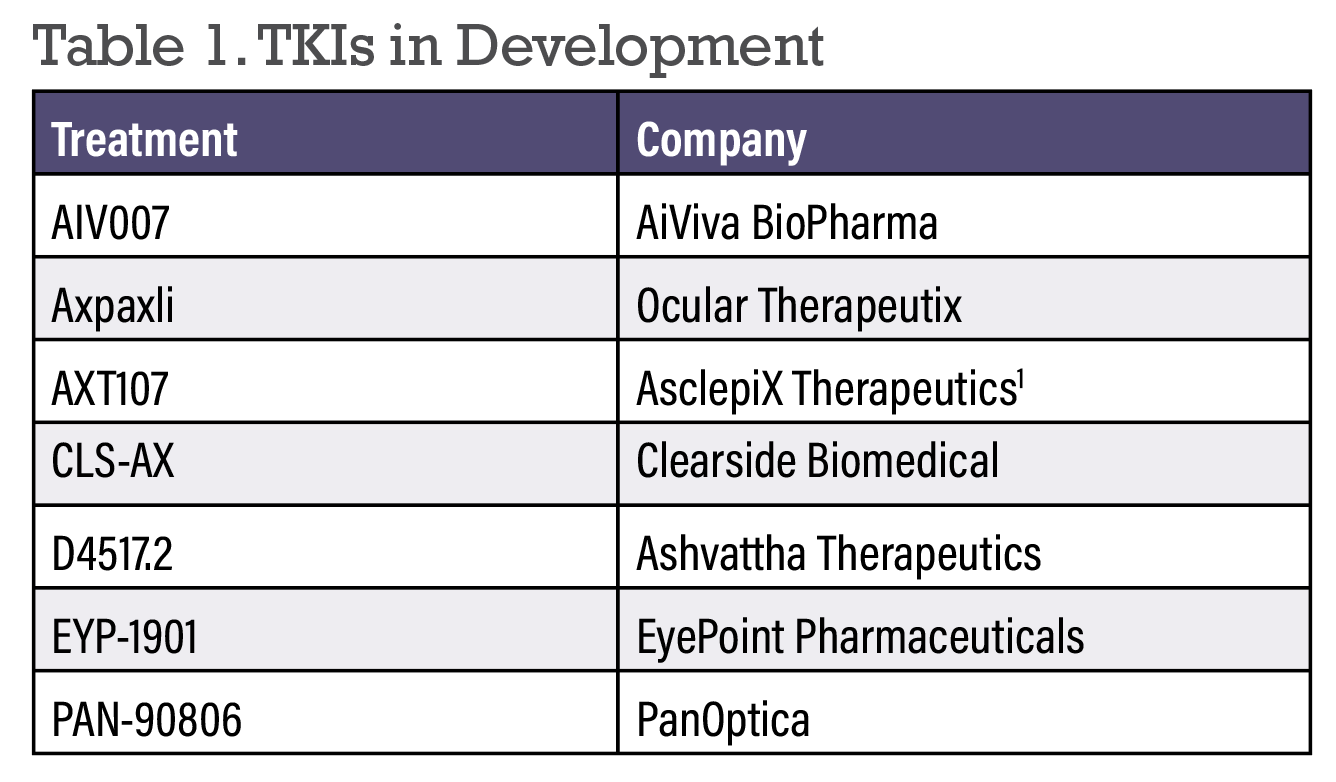 |
Whereas anti-VEGF medications primarily inhibit VEGF-A receptor binding extracellularly, TKIs are smaller molecules capable of diffusing into cells and inhibiting all VEGF receptor isoforms as well as other tyrosine kinase receptors such as FGFR and PDGFR.1,2 This versatility in terms of target inhibition allows the potential for enhanced durability and disease control. Below we’ll summarize the TKIs currently in development for wet AMD and DR (in alphabetical order).
• AIV007 (AiViva BioPharma). Relatively early in development, AIV007 is an injectable, broad-spectrum TKI that uses a proprietary technology that transitions to a biodissolvable gel depot at body temperature for prolonged drug release. A Phase I trial is enrolling 24 subjects with DME and wet AMD who’ll receive one periocular injection with monthly evaluation for up to six months. Study completion is set for 2025.
• Axpaxli (Ocular Therapeutix). Axpaxli (previously OTX-TKI) is the TKI axitinib delivered by Ocular Therapeutix’s Elutyx drug delivery platform. Elutyx technology is a completely bioresorbable, programmable hydrogel matrix that encapsulates axitinib to provide sustained and localized delivery lasting nine to 12 months with a single implant injected via a 25-gauge needle.3 Axitinib is a highly selective, pan-VEGF inhibitor offering the potential for higher potency and ocular cell biocompatibility than other TKIs.4 A Phase Ib randomized, double-masked trial evaluating wet AMD subjects with controlled retinal fluid compared 15 patients receiving 0.6 mg Axpaxli to five patients receiving aflibercept.5 Despite needing a mean of eight intravitreal injections in the year prior to initiation, Axpaxli patients enjoyed an 89-percent reduction in anti-VEGF treatment burden at 12 months, while vision and central subfield thickness (CST) values were comparable to standard-of-care aflibercept dosed every eight weeks. Seventy-three percent of patients needed no supplemental injections in the first six months, and 33 percent needed no injections at 12 months.
The HELIOS trial is a Phase Ib trial evaluating Axpaxli in moderate to severe NPDR patients without DME.6 This 12-month study should be releasing results very soon.
The SOL trial is a multicenter, double-masked, parallel-group, Phase III pivotal trial with 300 U.S. subjects comparing a reformulated, higher-dose Axpaxli to aflibercept in treatment-naïve wet AMD subjects with excellent visual acuity.7,8
• AXT107 (AsclepiX Therapeutics). AXT107 is a microparticulate suspension for intraocular injection that targets VEGF receptor 2 and activates the vessel-stabilizing receptor tyrosine kinase (TIE2). Fifteen patients are being recruited for a Phase I/IIa trial in wet AMD evaluating 40-week outcomes of three dose levels of a single injection.
• CLS-AX (Clearside Biomedical). CLS-AX is a proprietary formulation of the TKI axitinib that’s delivered via suprachoroidal administration using Clearside’s SCS Microinjector.9 As described above, Axitinib may have potency up to 10 times higher than other TKIs. Suprachoroidal administration offers a favorable safety and efficacy profile due to compartmentalization of medication closer to the target tissue, using the same microinjector as the FDA-approved Xipere.10
The OASIS Phase I/IIa extension trial evaluated escalating doses of CLS-AX following intravitreal aflibercept in previously treated wet AMD patients with active disease. Patients were evaluated monthly and could be retreated with aflibercept for 10 or greater letter loss with exudation, CST increase of 75 µm or new hemorrhage. The patients demonstrated stable visual acuity (BCVA from baseline) as well as CST values, while demonstrating a 77 to 85 percent reduction in treatment burden in cohorts 3 and 4 for up to six months. Sixty-seven percent of patients required no supplemental therapy out to six months, and CLS-AX displayed an excellent safety profile at all doses and timepoints.
Recruitment has subsequently been completed for the Phase IIb Odyssey trial evaluating 60 previously treated wet AMD patients with reading center confirmation of active disease. Both arms will receive three monthly aflibercept loading injections. Arm 1 will continue with aflibercept every eight weeks, while arm 2 receives CLS-AX at baseline and at least every 24 weeks. Patients are evaluated monthly at disease activity assessment (DAA) visits and can receive supplemental therapy if certain vision and anatomic criteria are met. These results, expected in the coming months, will help to inform a future Phase III development program.
• D4517.2 (Ashvattha Therapeutics). D4517.2 inhibits VEGF receptor tyrosine kinases selectively in activated microglia and macrophages and hypertrophic retinal pigment epithelial (RPE) cells. Preclinical studies have demonstrated that the agent crosses the blood-retinal barrier after systemic administration. Both oral and subcutaneous routes of administration are being explored.11 A single oral dose has shown reduction in CNV lesion size in mouse models, comparable to that provided by subcutaneous administration.12 The ongoing Phase II TEJAS study is evaluating the safety, efficacy and durability of multiple doses of subcutaneous D4517.2 compared to afibercept injections in previously treated wet AMD and DME patients.13
• EYP-1901 (EyePoint). EYP-1901 pairs vorolanib, a selected and patented TKI, with Eyepoint’s Durasert E technology. The Durasert E implant consists of drug embedded within a bioerodible matrix, providing an initial burst of drug followed by zero order kinetics and continuous, stable drug release for up to nine months via in-office intravitreal administration. In addition to pan-VEGF receptor inhibition, vorolanib also blocks PDGF, potentially providing antifibrotic benefits. Of note, it doesn’t inhibit TIE2 at clinically relevant doses. Vorolanib has also demonstrated neuroprotection in a validated retinal detachment animal model.
 |
On the heels of impressive durability results from the Phase I DAVIO trial, the DAVIO 2 trial is a multicenter, randomized, double-masked non-inferiority trial evaluating two doses of EYP-1901 against an aflibercept control in 160 previously treated wet AMD patients.14 Patients had received a mean of 10 injections in the year prior to screening. All three arms received three aflibercept loading doses. The control arm then was treated with aflibercept every eight weeks.15 Both the low- and high-dose EYP-1901 arms received the implant concomitantly with the third aflibercept loading dose, and could then receive supplemental anti-VEGF if certain vision and anatomic criteria were met, or at the investigators’ discretion.16
The primary endpoint was met with non-inferior change in BCVA compared with aflibercept monotherapy. There were no EYP-1901-related serious adverse events, and ocular AEs were mild and as expected for intravitreal injections. There were no cases of implant migration into the anterior chamber or occlusive retinal vasculitis. All secondary endpoints were met, including a greater than 80 percent reduction in treatment burden, both relative to the six months prior to study onset, as well as aflibercept control. Sixty-five percent of the 3-mg implant patients and 64 percent of the 2-mg patients were supplement-free six months after a single injection, while maintaining stable anatomy with OCT change at week 32 relative to aflibercept of less than 10 µm.
The PAVIA trial evaluating the vorolanib implant in moderate to severe non-proliferative diabetic retinopathy patients is ongoing with topline results expected soon.17 The VERONA trial is a single-masked, Phase II trial in previously treated DME patients. Lastly, a global Phase III trial in wet AMD is expected to commence shortly.
• PAN-90806 (PanOptica). PAN-90806 is a topical TKI eye drop studied in a Phase I and II, dose-escalating, quadruple-masked, randomized clinical trial evaluating once-daily use for 12 weeks in 51 treatment-naïve nAMD patients.18 Patient best-corrected acuity and CST remained stable, while also demonstrating a substantial 79-percent reduction in injection burden. Fifty-one percent of patients required no rescue injections. PanOptica has entered into a license agreement with Zhaoke Ophthalmology Pharmaceutical to fine tune the formulation for PAN-90806.19
Bottom Line
Highly encouraging results from numerous tyrosine kinase inhibitor clinical development programs are providing increasing levels of confidence that this new class of molecules may advance the treatment landscape for wet age-related macular degeneration and diabetic macular edema. Although patient outcomes have improved tremendously in the anti-VEGF era, challenges remain in terms of the need for frequent ongoing therapy in populations of elderly patients and diabetics, both of whom may struggle to maintain adherence to the prescribed treatment regimen.20
The promising candidates above may alleviate these obstacles, allowing improved long-term outcomes and quality of life for our patients. RS
1. Esteban-Villarrubia J, Soto-Castillo JJ, Pozas J, et al. Tyrosine kinase receptors in oncology. Int J Mol Sci. 2020;21:22:8529.
2. Vishwakarma S, Kaur I. Molecular mediators and regulators of retinal angiogenesis. Semin Ophthalmol. 2023;38:2:124-133.
3. Khanani AM, Couvillion SS, Eichenbaum DA, Steinle NC, Wykoff CC, Xavier S. 12-month update on randomized, controlled trial of OTX-TKI (axitinib intravitreal implant) for the treatment of wet AMD. Presented at: Clinical Trials at the Summit; June 10, 2023; Park City, UT.
4. Avery RL. OTX-TKI, sustained-release axitinib hydrogel implant, for neovascular age-related macular degeneration. Presented at: the Retina Society Annual Meeting; October 13, 2023; New York, NY.
5. Study evaluating the treatment of OTX-TKI for subjects with neovascular age-related macular degeneration. ClinicalTrials.gov identifier: NCT04989699. Updated September 6, 2022. Accessed May 20, 2024.
6. Study to evaluate the safety, tolerability, and efficacy of OTX-TKI in subjects with moderately severe to severe non-proliferative diabetic retinopathy. ClinicalTrials.gov identifier: NCT05695417. Updated December 8, 2023. Accessed May 20, 2024.
7. Ocular Therapeutix, Inc. Corporate presentation. November 2023. https://investors.ocutx.com/static-files/c8a4064b-8271-49db-ab5e-03a075777552. Accessed May 20, 2024.
8. Ocular Therapeutix, Inc. Ocular Therapeutix receives FDA agreement under special protocol assessment (SPA) for its first pivotal clinical trial of OTX-TKI in wet AMD. Press release. November 1, 2023. https://investors.ocutx.com/news-releases/news-release-details/ocular-therapeutixtm-receives-fda-agreement-under-special. Accessed May 20, 2024.
9. Clearside Biomedical, Inc. Corporate presentation. May 2023.. https://ir.clearsidebio.com/static-files/3c2f7b9f-cdd2-4a79-a22e-4d15471d57d2 Accessed May 20, 2024
10. Kansara VS, Muya LW, Ciulla TA. Evaluation of long-lasting potential of suprachoroidal axitinib suspension via ocular and systemic disposition in rabbits. Transl Vis Sci Technol. 2021;10:7:19. doi:10.1167/tvst.10.7.19
11. Ashvattha Therapeutics. D-4517.2 ophthalmology pipeline represents a paradigm shift in treatment. https://avttx.com/pipeline/ophthalmology/. Accessed May 20, 2024.
12. Le Moan N, Culp DW, Marjoram L, Dwadasi V, Cleland J. Oral formulation development of the anti-angiogenesis drug D-4517.2 to treat age-related macular degeneration (wet AMD) and diabetic macular edema (DME). Invest Ophthalmol Vis Sci. 2023;64:8:1299.
13. Ashvattha Therapeutics. Ashvattha Therapeutics announces first patient dosed via subcutaneous administration of anti-angiogenic therapeutic D-4517.2 for wet AMD and DME in phase 2 chronic dosing study. News release. November 1, 2023. https://avttx.com/ashvattha-therapeutics-announces-first-patient-dosed-via-subcutaneous-administration-of-anti-angiogenic-therapeutic-d-4517-2-for-wet-amd-and-dme-in-phase-2-chronic-dosing-study/. Accessed May 20, 2024.
14. Abbey AM, Patel S, Barakat MR, et al. The DAVIO trial: A phase 1, open-label, dose-escalation study of a single injection of EYP-1901 (vorolanib in durasert platform) demonstrating reduced treatment burden in wet age-related macular degeneration. Presented at: Association for Research in Vision and Ophthalmology; April 23-27,2023; New Orleans, LA.
15. Hershberger, V. The DAVIO2 trial: A Phase 2, multicenter study of a single injection of EYP-1901 (vorolanib in the durasert E technology) vs aflibercept for previously treated wet age-related macular degeneration. Presented at: Hawaiian Eye and Retina; January 13-19, 2024; Wailea, HI.
16. Study of EYP-1901 in subjects with wet age-related macular degeneration (wAMD) (DAVIO2). ClinicalTrials.gov identifier: NCT05381948. Updated July 19, 2023. Accessed May 20, 2024.
17. Study of EYP-1901 in patients with nonproliferative diabetic retinopathy (NPDR). ClinicalTrials.gov identifier: NCT05383209. Updated February 15, 2023. Accessed May 20, 2024.
18. Study of PAN-90806 eye drops, suspension for neovascular AMD. ClinicalTrials.gov identifier: NCT03479372. Updated July 9, 2019. Accessed May 20, 2024.
19. Zhaoke Hong Kong and PanOptica entered into license agreement for PAN-90806. News release. December 1, 2020. https://www.zkoph.com/news?lang=en&sid=38. Accessed May 20, 2024.
20. Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti–vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020; 4:1:19-30.



